American College of Cardiology03.18.19
In the year following placement of a CardioMEMS heart failure sensor—designed to wirelessly measure and monitor pulmonary artery pressures that can signal worsening heart failure—patients experienced a 58 percent reduction in hospitalization for heart failure, according to research presented at the American College of Cardiology’s 68th Annual Scientific Session. Reductions in hospitalizations were seen in both men and women, across all ejection fraction ranges and regardless of race.
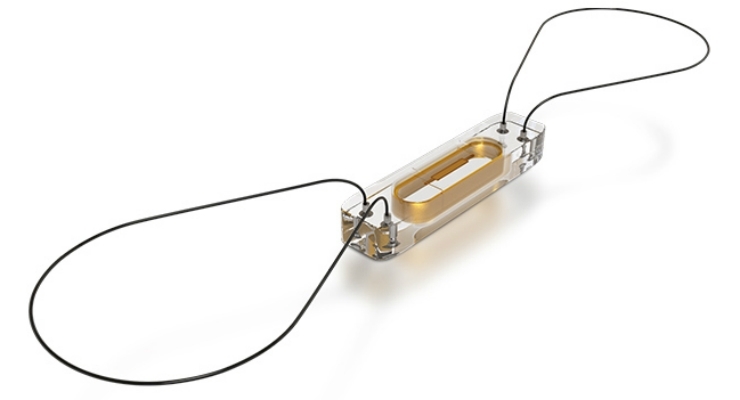
Heart failure, which affects nearly 6 million Americans, is a condition in which the heart cannot pump enough blood at the right pressures to meet the body’s needs. CardioMEMS is a small sensor—the size of a small paperclip—that is placed directly into a patient’s pulmonary artery, which connects the heart and the lungs. In a minimally invasive outpatient procedure, doctors use the femoral vein in the groin to thread the sensor up to the heart. Once implanted, the device can detect rising pressures in the pulmonary artery, which can be an early warning of fluid backing up in the lungs and pending onset of congestive heart failure even before symptoms of shortness of breath or weight gain are reported. Pressures are recorded and transmitted electronically from a patient’s home to a secure website so health care providers can review the readings and proactively adjust medical therapies to keep patients at their target pressures.
This prospective, open-label trial was initiated as a post-approval study to evaluate the efficacy and safety of the CardioMEMS sensor in clinical practice per U.S. Food and Drug Administration (FDA) mandates. The device was approved by the FDA in May 2014 for use in patients who have New York Heart Association (NYHA) Class III heart failure that limits daily life and who have been hospitalized for heart failure in the previous year. The study included 1,200 patients at 104 clinical sites in the U.S. Participants were an average of 69 years old and included 38 percent women, 17 percent non-white, 30 percent with preserved ejection fraction (HFpEF) and 53 percent with reduced ejection fraction.
“This study was done in a large number of patients with substantial representation of women and minorities and showed the device to be not only safe but markedly effective in keeping people out of the hospital,” said David Shavelle, M.D., associate professor at Keck School of Medicine of USC and the study’s lead author. “Our findings further validate the concept that remote monitoring of pulmonary artery pressures, which is a surrogate to a patients’ volume status, allows adjustment of medical therapy in a timely manner to prevent future heart failure hospitalizations. This represents an important advance in heart failure management, as these patients are at very high risk of hospitalizations and complications.”
The primary efficacy endpoint was heart failure hospitalization rates in the year after the sensor was implanted compared to the year before. Heart failure is among the top conditions that result in hospitalizations among people age 65 years and older. Patients in the study had an average of 1.24 heart failure hospitalizations in the year prior to implant and 0.52 hospitalizations in the year after having the device implanted. This translated to a 58 percent reduction in heart failure-related hospitalizations, researchers said. Similar reductions in hospitalizations were seen in patients with the greatest burden of hospitalizations (more than two hospitalizations in the previous year).
“Having the device cut the risk of hospitalizations by more than half,” Shavelle said. “The benefits of lower hospitalizations were seen across all subgroups of patients, and we also validated that this treatment can decrease hospitalizations in patients with HFpEF.”
The sensor prevented hospitalizations regardless of patients’ ejection fraction, preserved ejection fraction (50 percent or higher, which is considered normal), reduced ejection fraction (<40 percent) or mid-range ejection fraction (41-50 percent). Ejection fraction is a measure of how well the heart squeezes blood out of the heart to the body. There were also clear benefits for females and racial/ethnic minorities. Females had a 61 percent reduction in heart failure hospitalization and blacks had a reduction of 53 percent.
Additionally, patients with or without an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator and those with an ischemic or non-ischemic cardiomyopathy also saw lower rates of hospitalizations with the CardioMEMS sensor.
Moreover, having the device also appeared to reduce all-cause hospitalizations for conditions like pneumonia, chronic obstructive pulmonary disease or arrhythmias by 28 percent. Other analyses showed the combined rate of heart failure-related hospitalizations or death also dropped by 44 percent after the sensor was placed.
“If you can maintain more normal cardiac filling pressures and less heart stress, you are less likely to be seriously affected and need hospitalization for other conditions such as lung disease or liver disease, which are affected by heart function,” Shavelle said. “We believe that having the sensor monitored by their care team also encourages patients to follow their medication plan and gives them a sense of security that is particularly important for those living far away from a hospital.”
The CardioMEMS sensor also met its safety endpoint—freedom from device or system-related complications or sensor failure at one year. To assess safety, researchers tracked whether there were any device or system-related complications and episodes of sensor failure where they were unable to get pressure readings from the device even after troubleshooting the external electronics. Based on the data, only four patients had device- or system-related complications, and there was only one episode of sensor failure, Shavelle said. Reported another way, at one year post-implant, study participants had 99.7 percent freedom from device/system-related complications and 99.9 percent freedom from sensor failure.
An ongoing study is evaluating the use of the CardioMEMS sensor for patients with other classes of heart failure (NYHA Class II and IV) and for patients at risk but without a prior hospitalization for heart failure.
This study was funded by Abbott Vascular.
Heart failure, which affects nearly 6 million Americans, is a condition in which the heart cannot pump enough blood at the right pressures to meet the body’s needs. CardioMEMS is a small sensor—the size of a small paperclip—that is placed directly into a patient’s pulmonary artery, which connects the heart and the lungs. In a minimally invasive outpatient procedure, doctors use the femoral vein in the groin to thread the sensor up to the heart. Once implanted, the device can detect rising pressures in the pulmonary artery, which can be an early warning of fluid backing up in the lungs and pending onset of congestive heart failure even before symptoms of shortness of breath or weight gain are reported. Pressures are recorded and transmitted electronically from a patient’s home to a secure website so health care providers can review the readings and proactively adjust medical therapies to keep patients at their target pressures.
This prospective, open-label trial was initiated as a post-approval study to evaluate the efficacy and safety of the CardioMEMS sensor in clinical practice per U.S. Food and Drug Administration (FDA) mandates. The device was approved by the FDA in May 2014 for use in patients who have New York Heart Association (NYHA) Class III heart failure that limits daily life and who have been hospitalized for heart failure in the previous year. The study included 1,200 patients at 104 clinical sites in the U.S. Participants were an average of 69 years old and included 38 percent women, 17 percent non-white, 30 percent with preserved ejection fraction (HFpEF) and 53 percent with reduced ejection fraction.
“This study was done in a large number of patients with substantial representation of women and minorities and showed the device to be not only safe but markedly effective in keeping people out of the hospital,” said David Shavelle, M.D., associate professor at Keck School of Medicine of USC and the study’s lead author. “Our findings further validate the concept that remote monitoring of pulmonary artery pressures, which is a surrogate to a patients’ volume status, allows adjustment of medical therapy in a timely manner to prevent future heart failure hospitalizations. This represents an important advance in heart failure management, as these patients are at very high risk of hospitalizations and complications.”
The primary efficacy endpoint was heart failure hospitalization rates in the year after the sensor was implanted compared to the year before. Heart failure is among the top conditions that result in hospitalizations among people age 65 years and older. Patients in the study had an average of 1.24 heart failure hospitalizations in the year prior to implant and 0.52 hospitalizations in the year after having the device implanted. This translated to a 58 percent reduction in heart failure-related hospitalizations, researchers said. Similar reductions in hospitalizations were seen in patients with the greatest burden of hospitalizations (more than two hospitalizations in the previous year).
“Having the device cut the risk of hospitalizations by more than half,” Shavelle said. “The benefits of lower hospitalizations were seen across all subgroups of patients, and we also validated that this treatment can decrease hospitalizations in patients with HFpEF.”
The sensor prevented hospitalizations regardless of patients’ ejection fraction, preserved ejection fraction (50 percent or higher, which is considered normal), reduced ejection fraction (<40 percent) or mid-range ejection fraction (41-50 percent). Ejection fraction is a measure of how well the heart squeezes blood out of the heart to the body. There were also clear benefits for females and racial/ethnic minorities. Females had a 61 percent reduction in heart failure hospitalization and blacks had a reduction of 53 percent.
Additionally, patients with or without an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator and those with an ischemic or non-ischemic cardiomyopathy also saw lower rates of hospitalizations with the CardioMEMS sensor.
Moreover, having the device also appeared to reduce all-cause hospitalizations for conditions like pneumonia, chronic obstructive pulmonary disease or arrhythmias by 28 percent. Other analyses showed the combined rate of heart failure-related hospitalizations or death also dropped by 44 percent after the sensor was placed.
“If you can maintain more normal cardiac filling pressures and less heart stress, you are less likely to be seriously affected and need hospitalization for other conditions such as lung disease or liver disease, which are affected by heart function,” Shavelle said. “We believe that having the sensor monitored by their care team also encourages patients to follow their medication plan and gives them a sense of security that is particularly important for those living far away from a hospital.”
The CardioMEMS sensor also met its safety endpoint—freedom from device or system-related complications or sensor failure at one year. To assess safety, researchers tracked whether there were any device or system-related complications and episodes of sensor failure where they were unable to get pressure readings from the device even after troubleshooting the external electronics. Based on the data, only four patients had device- or system-related complications, and there was only one episode of sensor failure, Shavelle said. Reported another way, at one year post-implant, study participants had 99.7 percent freedom from device/system-related complications and 99.9 percent freedom from sensor failure.
An ongoing study is evaluating the use of the CardioMEMS sensor for patients with other classes of heart failure (NYHA Class II and IV) and for patients at risk but without a prior hospitalization for heart failure.
This study was funded by Abbott Vascular.