Business Wire02.14.19
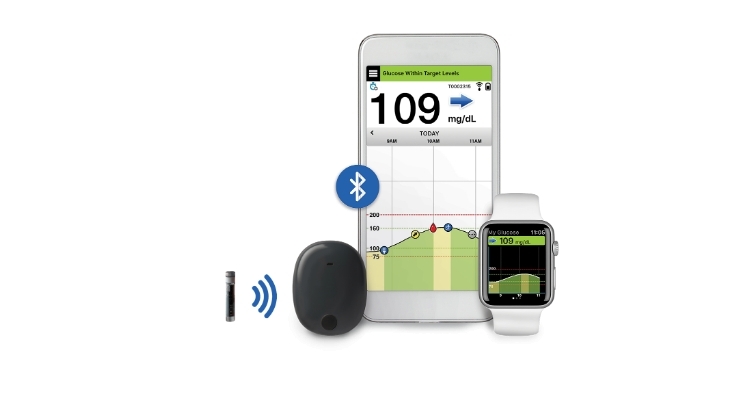
Senseonics Holdings Inc., a medical technology company focused on the development and commercialization of a long-term, implantable continuous glucose monitoring (CGM) system for people with diabetes, announced that the extended life Eversense XL sensor that lasts up to 180 days has been implanted in the first U.S. study participant as part of the clinical trial for pre-market application submission to the U.S. Food and Drug Administration (FDA).
The PROMISE clinical study is intended to evaluate the safety and efficacy of the Eversense CGM system in people with diabetes over a 180-day period. Approximately 180 study participants at up to 15 locations across the United States are planned to enroll in the study. The Eversense XL sensor previously received the CE Mark and is currently marketed to patients across the European Union.
Senseonics also announced that the company has completed its submission of PMA supplements to the FDA to secure an insulin dosing claim and to remove the contraindication related to the magnetic resonance imaging (MRI) exposure on the 90-day system which is currently available in the United States.
“I am thrilled to be able to offer a long term implantable sensor to my patients through the PROMISE 180-day clinical study,” said Dr. Mark Christiansen, co-medical director of Diablo Clinical Research and the first physician to insert the extended life long-term sensor. “We are looking forward to providing patients six months of continuous sensing and the potential benefits of a long-term sensor.”
“We are pleased to have enrolled the first participant in this important study which demonstrates our continued progress in transforming CGM technology. This is the first study in the US in which participants are implanted with a single sensor designed to produce accurate continuous glucose measurements for half of a year,” said Tim Goodnow, Senseonics president and CEO. “Our submission of the supplements to secure the dosing claim and to remove the MRI contraindication for the current 90-Day Eversense system are significant step forward in reducing the burden of diabetes management and providing patients peace of mind.”
Senseonics Holdings Inc. is a medical technology company focused on the design, development, and commercialization of transformative glucose monitoring products designed to help people with diabetes confidently live their lives with ease. From its inception, Senseonics has been advancing the integration of novel, fluorescence sensor technology with smart wearable devices. The Eversense CGM System received PMA approval from the FDA for up to 90 days of continuous use and is available in the United States. The Eversense XL CGM System received CE mark for up to 180 days of continuous use and is available in Europe.
The PROMISE clinical study is intended to evaluate the safety and efficacy of the Eversense CGM system in people with diabetes over a 180-day period. Approximately 180 study participants at up to 15 locations across the United States are planned to enroll in the study. The Eversense XL sensor previously received the CE Mark and is currently marketed to patients across the European Union.
Senseonics also announced that the company has completed its submission of PMA supplements to the FDA to secure an insulin dosing claim and to remove the contraindication related to the magnetic resonance imaging (MRI) exposure on the 90-day system which is currently available in the United States.
“I am thrilled to be able to offer a long term implantable sensor to my patients through the PROMISE 180-day clinical study,” said Dr. Mark Christiansen, co-medical director of Diablo Clinical Research and the first physician to insert the extended life long-term sensor. “We are looking forward to providing patients six months of continuous sensing and the potential benefits of a long-term sensor.”
“We are pleased to have enrolled the first participant in this important study which demonstrates our continued progress in transforming CGM technology. This is the first study in the US in which participants are implanted with a single sensor designed to produce accurate continuous glucose measurements for half of a year,” said Tim Goodnow, Senseonics president and CEO. “Our submission of the supplements to secure the dosing claim and to remove the MRI contraindication for the current 90-Day Eversense system are significant step forward in reducing the burden of diabetes management and providing patients peace of mind.”
Senseonics Holdings Inc. is a medical technology company focused on the design, development, and commercialization of transformative glucose monitoring products designed to help people with diabetes confidently live their lives with ease. From its inception, Senseonics has been advancing the integration of novel, fluorescence sensor technology with smart wearable devices. The Eversense CGM System received PMA approval from the FDA for up to 90 days of continuous use and is available in the United States. The Eversense XL CGM System received CE mark for up to 180 days of continuous use and is available in Europe.