PR Newswire01.03.19
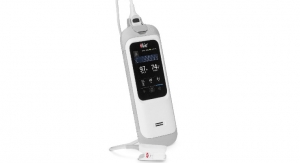
Bruin Biometrics, LLC ("BBI") has been granted U.S. Food and Drug Administration (FDA) marketing authorization for the SEM Scanner, a wireless handheld device that is indicated for use as an adjunct to the standard of care when assessing patients who are at increased risk for pressure ulcers.
The SEM Scanner is the world's first FDA-authorized device to objectively alert clinicians to specific anatomical areas of a patient's body at increased risk for developing pressure damage. Patient risk assessments are performed with the SEM Scanner before visible damage manifests at the skin surface—a world and clinical first.
Current clinical decision making relies on assessing a patient's overall risk for pressure ulcer development and then completing a subjective skin and tissue assessment. Both methods suffer from not being able to direct clinicians to where the risk is building until damage is visible at the skin's surface. Once pressure injuries (also known as pressure ulcers, or bed sores) become visible on the skin's surface, tissue damage has already occurred2.
Elevated readings from the SEM Scanner provide objective clinical information that directs clinicians to heels and sacrums at risk of developing pressure ulcers, even before the damage is visible. These data can facilitate earlier, anatomically specific interventions designed to reverse the damaging effects of pressure and shear and prevent the pressure injuries from breaking through the skin.
Still the number one most reported patient safety harm in many U.S. states, pressure ulcer incidence has remained stubbornly persistent3. More than 2.5 million people annually develop bed sores in the United States, including nearly one out of 10 patients in hospitals and almost one-third of patients in long-term acute care4.
Bed sores can lead to pain, disfigurement, infection, and complications such as sepsis, cellulitis, and MRSA. 60,000 Americans die each year4 from complications from pressure ulcers—an equivalent mortality rate to the opioid crisis5—at an annual cost of up to $11.6 billion to the U.S. health care system6. These injuries result from pressure and shear causing localized damage to the skin and underlying tissue, typically at areas of bony prominence, such as the heels and sacrum.
Outside the United States, clinicians have been using the SEM Scanner in conjunction with existing risk assessment tools since 2014. U.K. NHS and private providers have enjoyed and won numerous awards for innovation and patient safety, including the Health Service Journal's Patient Safety Award for Innovation (2017)7 and the Journal of Wound Care's Most Innovative Product (2018)8 for their use of the SEM Scanner.
Valuable Advance Notice
FDA granted marketing authorization for the SEM Scanner under its de novo review process for novel low- to moderate-risk devices that are not substantially equivalent to an already legally marketed device.
FDA authorization was based, in part, on data from a clinical study assessing performance of the SEM Scanner compared to visual skin assessment by nurses in 182 patients at risk for pressure ulcers at 12 hospitals and skilled nursing facilities in the U.S. and United Kingdom9.
The SEM Scanner received European CE mark approval in 2014 and Health Canada clearance in 2016 and is in full commercial use in Canada and the European Union.
No adverse or serious adverse events have occurred from SEM Scanner use.
"Having anatomically specific risk information gives nurses valuable advance notice to institute additional preventive treatment tailored to a patient's unique needs," said Dr. Ruth Bryant, a SEM Scanner study investigator, author of "Acute & Chronic Wounds" (2016), certified wound ostomy continence nurse, director of nursing research at Abbott Northwestern Hospital in Minneapolis and president-elect of the Association for the Advancement of Wound Care.
"The anatomically specific information combined with tailored preventive actions may ultimately translate into fewer pressure sores, decreased costs, increased quality of patient care, increased patient satisfaction and decreased risk for adverse events due to pressure ulcers such as in-hospital mortality, prolonged length of stay, discharge to an extended care facility rather than to the home, and infection," Dr. Bryant added.
Dr. Barbara Bates-Jensen, a professor at the UCLA School of Nursing and the David Geffen School of Medicine and a co-inventor of the SEM Scanner, said: "Objective, scientific data from the SEM Scanner can give clinicians confidence to take action and intervene with methods to prevent pressure ulcers."
Representative Ted W. Lieu (D-CA), representing the Los Angeles district where BBI is based, stated: "With the SEM Scanner, nurses have a new tool to risk assess patients for injuries that disproportionately impact the elderly, disabled veterans and others with limited mobility."
BBI CEO Martin Burns welcomed FDA's decision: "Our singular objective is to reduce pressure injury incidence by helping clinicians make prevention real. Total prevention of avoidable pressure injuries is mathematically impossible under the current standard of care. Prevention success demands objective, early, anatomically specific data. For the first time, clinicians will have access to anatomically specific risk assessment data that can be gathered from increased risk patients in all care settings. We are optimistic about the impact these data will have on prevention strategies here in the U.S.A. With this FDA decision, wound prevention has finally caught up with other areas of healthcare that have long benefited from medical technologies."
Burns continued, "We have very carefully recruited talented and experienced sales leaders, implementation and education professionals for the U.S. market. Approximately 20 U.S. and EU professionals are scheduled to attend formal office and field-based training in the U.K. in mid-January 2019."
References
1 Glenn Smith R.N., Clinical Nurse Specialist Nutrition and Tissue Viability at Isle of Wight NHS Trust, St. Mary's Hospital, Isle of Wight
2 Oomens CW1, Bader DL, Loerakker S, Baaijens F. (2015) Pressure induced deep tissue injury explained. Ann Biomed Eng.Feb;43(2):297-305. doi: 10.1007/s10439-014-1202-6. Epub 2014 Dec 6.
3 Padula, W., et al (2018) Value of hospital resources for effective pressure injury prevention: a cost-effectiveness analysis. BMJ Qual Saf. 0:1-10 doi:10.1136/bmjqs-2017-007505
4 Preventing Pressure Ulcers in Hospitals. (2014, October 02). Retrieved from https://www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/index.html
5 https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
6 Russo CA, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers among Adults 18 Years and Older, 2006. Healthcare Cost and Utilization Project (HCUP. Rockville, MD: Agency for Healthcare Research and Quality, 2008.
7 https://awards.patientsafetycongress.co.uk/patient-safety-awards-winners-2017-1
8 https://www.jwcawards.com/winners
9 Evaluation of a Novel Device Using Capacitance of the Detection of Early Pressure Ulcers (PU), a Multi-Site Longitudinal Study. Okonkwo H. Milne et al. (2018). Accepted and presented at NPUAP 2018. Retrieved from https://epostersonline.com/wounds2017/node/814
The SEM Scanner is the world's first FDA-authorized device to objectively alert clinicians to specific anatomical areas of a patient's body at increased risk for developing pressure damage. Patient risk assessments are performed with the SEM Scanner before visible damage manifests at the skin surface—a world and clinical first.
Current clinical decision making relies on assessing a patient's overall risk for pressure ulcer development and then completing a subjective skin and tissue assessment. Both methods suffer from not being able to direct clinicians to where the risk is building until damage is visible at the skin's surface. Once pressure injuries (also known as pressure ulcers, or bed sores) become visible on the skin's surface, tissue damage has already occurred2.
Elevated readings from the SEM Scanner provide objective clinical information that directs clinicians to heels and sacrums at risk of developing pressure ulcers, even before the damage is visible. These data can facilitate earlier, anatomically specific interventions designed to reverse the damaging effects of pressure and shear and prevent the pressure injuries from breaking through the skin.
Still the number one most reported patient safety harm in many U.S. states, pressure ulcer incidence has remained stubbornly persistent3. More than 2.5 million people annually develop bed sores in the United States, including nearly one out of 10 patients in hospitals and almost one-third of patients in long-term acute care4.
Bed sores can lead to pain, disfigurement, infection, and complications such as sepsis, cellulitis, and MRSA. 60,000 Americans die each year4 from complications from pressure ulcers—an equivalent mortality rate to the opioid crisis5—at an annual cost of up to $11.6 billion to the U.S. health care system6. These injuries result from pressure and shear causing localized damage to the skin and underlying tissue, typically at areas of bony prominence, such as the heels and sacrum.
Outside the United States, clinicians have been using the SEM Scanner in conjunction with existing risk assessment tools since 2014. U.K. NHS and private providers have enjoyed and won numerous awards for innovation and patient safety, including the Health Service Journal's Patient Safety Award for Innovation (2017)7 and the Journal of Wound Care's Most Innovative Product (2018)8 for their use of the SEM Scanner.
Valuable Advance Notice
FDA granted marketing authorization for the SEM Scanner under its de novo review process for novel low- to moderate-risk devices that are not substantially equivalent to an already legally marketed device.
FDA authorization was based, in part, on data from a clinical study assessing performance of the SEM Scanner compared to visual skin assessment by nurses in 182 patients at risk for pressure ulcers at 12 hospitals and skilled nursing facilities in the U.S. and United Kingdom9.
The SEM Scanner received European CE mark approval in 2014 and Health Canada clearance in 2016 and is in full commercial use in Canada and the European Union.
No adverse or serious adverse events have occurred from SEM Scanner use.
"Having anatomically specific risk information gives nurses valuable advance notice to institute additional preventive treatment tailored to a patient's unique needs," said Dr. Ruth Bryant, a SEM Scanner study investigator, author of "Acute & Chronic Wounds" (2016), certified wound ostomy continence nurse, director of nursing research at Abbott Northwestern Hospital in Minneapolis and president-elect of the Association for the Advancement of Wound Care.
"The anatomically specific information combined with tailored preventive actions may ultimately translate into fewer pressure sores, decreased costs, increased quality of patient care, increased patient satisfaction and decreased risk for adverse events due to pressure ulcers such as in-hospital mortality, prolonged length of stay, discharge to an extended care facility rather than to the home, and infection," Dr. Bryant added.
Dr. Barbara Bates-Jensen, a professor at the UCLA School of Nursing and the David Geffen School of Medicine and a co-inventor of the SEM Scanner, said: "Objective, scientific data from the SEM Scanner can give clinicians confidence to take action and intervene with methods to prevent pressure ulcers."
Representative Ted W. Lieu (D-CA), representing the Los Angeles district where BBI is based, stated: "With the SEM Scanner, nurses have a new tool to risk assess patients for injuries that disproportionately impact the elderly, disabled veterans and others with limited mobility."
BBI CEO Martin Burns welcomed FDA's decision: "Our singular objective is to reduce pressure injury incidence by helping clinicians make prevention real. Total prevention of avoidable pressure injuries is mathematically impossible under the current standard of care. Prevention success demands objective, early, anatomically specific data. For the first time, clinicians will have access to anatomically specific risk assessment data that can be gathered from increased risk patients in all care settings. We are optimistic about the impact these data will have on prevention strategies here in the U.S.A. With this FDA decision, wound prevention has finally caught up with other areas of healthcare that have long benefited from medical technologies."
Burns continued, "We have very carefully recruited talented and experienced sales leaders, implementation and education professionals for the U.S. market. Approximately 20 U.S. and EU professionals are scheduled to attend formal office and field-based training in the U.K. in mid-January 2019."
References
1 Glenn Smith R.N., Clinical Nurse Specialist Nutrition and Tissue Viability at Isle of Wight NHS Trust, St. Mary's Hospital, Isle of Wight
2 Oomens CW1, Bader DL, Loerakker S, Baaijens F. (2015) Pressure induced deep tissue injury explained. Ann Biomed Eng.Feb;43(2):297-305. doi: 10.1007/s10439-014-1202-6. Epub 2014 Dec 6.
3 Padula, W., et al (2018) Value of hospital resources for effective pressure injury prevention: a cost-effectiveness analysis. BMJ Qual Saf. 0:1-10 doi:10.1136/bmjqs-2017-007505
4 Preventing Pressure Ulcers in Hospitals. (2014, October 02). Retrieved from https://www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/index.html
5 https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
6 Russo CA, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers among Adults 18 Years and Older, 2006. Healthcare Cost and Utilization Project (HCUP. Rockville, MD: Agency for Healthcare Research and Quality, 2008.
7 https://awards.patientsafetycongress.co.uk/patient-safety-awards-winners-2017-1
8 https://www.jwcawards.com/winners
9 Evaluation of a Novel Device Using Capacitance of the Detection of Early Pressure Ulcers (PU), a Multi-Site Longitudinal Study. Okonkwo H. Milne et al. (2018). Accepted and presented at NPUAP 2018. Retrieved from https://epostersonline.com/wounds2017/node/814