Business Wire11.07.18
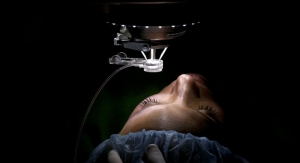
LuxCath LLC, a medical device company developing a cardiac catheter ablation technology, has appointed Terry Ransbury as chief technology officer. Ransbury is a seasoned industry veteran who brings over 30 years of experience in research and development, specializing in electrophysiology and cardiac rhythm management. He will spearhead the development and commercialization of LuxCath’s proprietary OmniView light-guided catheter ablation system, the only technology to see into tissue during cardiac ablation for assessing catheter-tissue contact and lesion progression in real-time. The OmniView technology provides electrophysiologists with a simple, definitive endpoint for lesion quality, thus increasing the potential for complete and more rapid, effective cardiac ablation.
“We are thrilled to have someone of Terry’s caliber lead our development efforts and help us fully realize the potential of our breakthrough light-guided ablation technology,” said Omar Amirana, M.D., CEO of LuxCath. “Terry’s extensive experience in research and development, as well as long-standing relationships with leading electrophysiologists, will be important as we focus on our immediate goal of bringing our technology to market.”
“I believe LuxCath has the transformative science and encouraging data to revolutionize the treatment of cardiac arrhythmias,” said Ransbury. “I have worked with this technology since its inception and am confident of its potential to improve patient outcomes, reduce procedure times and costly repeat ablations. The electrophysiology market stands to significantly benefit from this remarkable breakthrough and I am thrilled to join LuxCath as its first full-time employee.”
Ransbury has participated in over 600 human cardiac ablation procedures around the world and is EP-lab certified. He has several years of industry experience, having previously served at startups, such as Ventritex (now part of St. Jude Medical/Abbott) and Biosense (now part of Johnson & Johnson). He has a bachelor’s degree in electrical engineering and biomedical engineering from Duke University and is listed as the inventor on over 40 U.S. patents and patent applications. Most recently, Ransbury served as co-founder and president at Nocturnal Product Development, a medical device development firm, where he was responsible for new business development, system architecture design, and project management. Nocturnal Product Development has served as LuxCath’s product development partner since inception and has been instrumental in developing the company’s core technology to date.
“Although we are sad to see Terry leave Nocturnal, I am extremely proud of the progress we have made with LuxCath and welcome the prospect of continuing our partnership and seeing through the development of this hugely innovative technology,” said K.C. Armstrong, co-founder and newly appointed president at Nocturnal Product Development.
Separately, LuxCath has appointed to its board Rick Randall and John Fletcher. The appointments are effective immediately and increases the size of LuxCath’s board to five members.
“Rick and John are two highly regarded industry veterans and their appointments to our board of directors reflects the disruptive potential we believe our technology offers electrophysiologists,” said Amirana. “Rick and John will both be instrumental in providing valuable insights regarding our operations, strategy, and corporate governance. We are delighted to welcome them both and look forward to leveraging their knowledge as we move towards a CE Mark and EU commercialization.”
Randall has over 40 years of experience in the medical device industry and has held various leadership and board positions. Most recently, Randall served as CEO of OMNIlife Science Inc., the first company to commercialize robotic-assisted total knee replacement in the United States. Before OMNI, he served as CEO of Innovasive Devices Inc., acquired by Johnson & Johnson in 2000, and Target Therapeutics Inc., acquired by Boston Scientific Corp. in 1997. In addition, Randall currently serves as a board advisor at RadiaDyne LLC and director at Fortus Medical Inc. Randall has previously served on the boards of several medical device companies, including Endocardial Solutions Inc. and Conceptus Inc.
Fletcher serves as the CEO and managing director of Fletcher Spaght Inc., which he founded in 1983. He is a founding partner and managing director at Fletcher Spaght Ventures LP. Prior to launching Fletcher Spaght Inc., he served as a senior manager at The Boston Consulting Group, managing client relationships in healthcare and high technology companies. Fletcher has extensive work experience in strategic planning, especially in the area of market analysis for technology-based businesses. He served as board chairman of The Spectranetics Corporation from December 2010 until its acquisition by Philips in August 2017. For his work at Spectranetics, he was named Director of the Year by the National Association of Corporate Directors. He currently serves on the boards of Axcelis Technologies Inc., MRI Interventions Inc., and Metabolon. He is also on the Boards of Advisors of the Whitehead Institute at the Massachusetts Institute of Technology, the Thayer School of Engineering at Dartmouth College, and the School of Government and Business at The George Washington University. Fletcher previously served on the boards of NMT Medical, Marina Biotech Inc., AutoImmune, and Cayenne Software Inc. Fletcher was a captain and fighter pilot in the U.S. Air Force.
LuxCath is a medical technology company founded to address persistent unmet needs in the catheter ablation treatment of cardiac arrhythmias. The company has developed technology that uses fluorescence and optics to directly view lesions during cardiac ablation procedures. Its flagship product, the OmniView light-guided ablation catheter, allows electrophysiologists for the first time to see and assess catheter-tissue contact quality, tissue composition and lesion quality in real time during the procedure, increasing the potential for complete and effective cardiac ablation.
Nocturnal Product Development LLC is a medical device development firm located near Research Triangle Park, Durham, N.C. The company specializes in multi-disciplinary technology product development with expertise in: electrical design, mechanical design, embedded software, optics, IOT, wireless, cloud-based applications, project management, and clinical & regulatory strategy.
Allied Minds is an innovative U.S. science and technology development and commercialization company based in Boston, Mass. Operating since 2006, Allied Minds forms, funds, manages and builds products and businesses based on technologies developed at U.S. universities and federal research institutions. Allied Minds serves as a diversified holding company that supports its businesses and product development with capital, central management and shared services.
“We are thrilled to have someone of Terry’s caliber lead our development efforts and help us fully realize the potential of our breakthrough light-guided ablation technology,” said Omar Amirana, M.D., CEO of LuxCath. “Terry’s extensive experience in research and development, as well as long-standing relationships with leading electrophysiologists, will be important as we focus on our immediate goal of bringing our technology to market.”
“I believe LuxCath has the transformative science and encouraging data to revolutionize the treatment of cardiac arrhythmias,” said Ransbury. “I have worked with this technology since its inception and am confident of its potential to improve patient outcomes, reduce procedure times and costly repeat ablations. The electrophysiology market stands to significantly benefit from this remarkable breakthrough and I am thrilled to join LuxCath as its first full-time employee.”
Ransbury has participated in over 600 human cardiac ablation procedures around the world and is EP-lab certified. He has several years of industry experience, having previously served at startups, such as Ventritex (now part of St. Jude Medical/Abbott) and Biosense (now part of Johnson & Johnson). He has a bachelor’s degree in electrical engineering and biomedical engineering from Duke University and is listed as the inventor on over 40 U.S. patents and patent applications. Most recently, Ransbury served as co-founder and president at Nocturnal Product Development, a medical device development firm, where he was responsible for new business development, system architecture design, and project management. Nocturnal Product Development has served as LuxCath’s product development partner since inception and has been instrumental in developing the company’s core technology to date.
“Although we are sad to see Terry leave Nocturnal, I am extremely proud of the progress we have made with LuxCath and welcome the prospect of continuing our partnership and seeing through the development of this hugely innovative technology,” said K.C. Armstrong, co-founder and newly appointed president at Nocturnal Product Development.
Separately, LuxCath has appointed to its board Rick Randall and John Fletcher. The appointments are effective immediately and increases the size of LuxCath’s board to five members.
“Rick and John are two highly regarded industry veterans and their appointments to our board of directors reflects the disruptive potential we believe our technology offers electrophysiologists,” said Amirana. “Rick and John will both be instrumental in providing valuable insights regarding our operations, strategy, and corporate governance. We are delighted to welcome them both and look forward to leveraging their knowledge as we move towards a CE Mark and EU commercialization.”
Randall has over 40 years of experience in the medical device industry and has held various leadership and board positions. Most recently, Randall served as CEO of OMNIlife Science Inc., the first company to commercialize robotic-assisted total knee replacement in the United States. Before OMNI, he served as CEO of Innovasive Devices Inc., acquired by Johnson & Johnson in 2000, and Target Therapeutics Inc., acquired by Boston Scientific Corp. in 1997. In addition, Randall currently serves as a board advisor at RadiaDyne LLC and director at Fortus Medical Inc. Randall has previously served on the boards of several medical device companies, including Endocardial Solutions Inc. and Conceptus Inc.
Fletcher serves as the CEO and managing director of Fletcher Spaght Inc., which he founded in 1983. He is a founding partner and managing director at Fletcher Spaght Ventures LP. Prior to launching Fletcher Spaght Inc., he served as a senior manager at The Boston Consulting Group, managing client relationships in healthcare and high technology companies. Fletcher has extensive work experience in strategic planning, especially in the area of market analysis for technology-based businesses. He served as board chairman of The Spectranetics Corporation from December 2010 until its acquisition by Philips in August 2017. For his work at Spectranetics, he was named Director of the Year by the National Association of Corporate Directors. He currently serves on the boards of Axcelis Technologies Inc., MRI Interventions Inc., and Metabolon. He is also on the Boards of Advisors of the Whitehead Institute at the Massachusetts Institute of Technology, the Thayer School of Engineering at Dartmouth College, and the School of Government and Business at The George Washington University. Fletcher previously served on the boards of NMT Medical, Marina Biotech Inc., AutoImmune, and Cayenne Software Inc. Fletcher was a captain and fighter pilot in the U.S. Air Force.
LuxCath is a medical technology company founded to address persistent unmet needs in the catheter ablation treatment of cardiac arrhythmias. The company has developed technology that uses fluorescence and optics to directly view lesions during cardiac ablation procedures. Its flagship product, the OmniView light-guided ablation catheter, allows electrophysiologists for the first time to see and assess catheter-tissue contact quality, tissue composition and lesion quality in real time during the procedure, increasing the potential for complete and effective cardiac ablation.
Nocturnal Product Development LLC is a medical device development firm located near Research Triangle Park, Durham, N.C. The company specializes in multi-disciplinary technology product development with expertise in: electrical design, mechanical design, embedded software, optics, IOT, wireless, cloud-based applications, project management, and clinical & regulatory strategy.
Allied Minds is an innovative U.S. science and technology development and commercialization company based in Boston, Mass. Operating since 2006, Allied Minds forms, funds, manages and builds products and businesses based on technologies developed at U.S. universities and federal research institutions. Allied Minds serves as a diversified holding company that supports its businesses and product development with capital, central management and shared services.