GlobeNewswire07.16.18
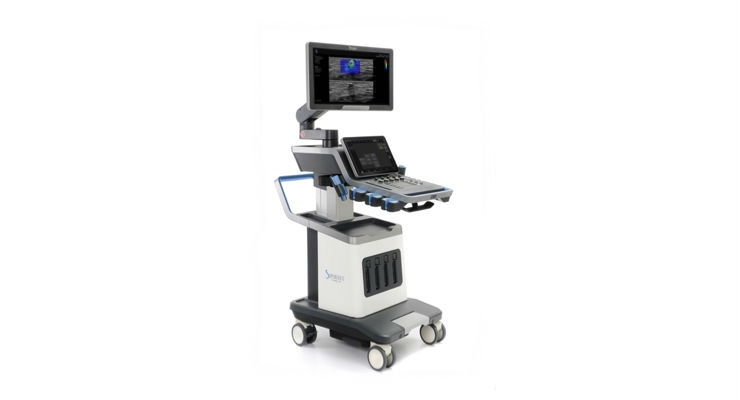
SuperSonic Imagine, a company specialized in ultrasound medical imaging, today announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) and authorization to use the CE marking from the LNE/GMed for its new cutting-edge smart ultrasound system, the Aixplorer MACH 30.
“The FDA 510(k) and CE mark are critical milestones in the launch of our exciting new system,” explains Michèle Lesieur, SuperSonic Imagine CEO and Chairwoman of its Board of Directors. “These approvals not only confirm that the Aixplorer MACH 30 is recognized for its quality, performance, and compliance with American and European regulatory standards, but it also clears the way for marketing of the system in the US, European member states, and many other countries around the world. We are confident this new innovative platform will offer clinical value which supports our growth ambitions in the Global Ultrasound Market.”
The new system represents another technological breakthrough from SuperSonic Imagine. Aixplorer MACH 30 offers the latest UltraFast imaging, enhancing a full range of innovative imaging modes developed by SuperSonic Imagine for superior diagnostic performance.
The greater performance, combined with an elegant and functional design and streamlined user experience of the Aixplorer MACH 30 will win over users around the world. It includes the SonicPad touchpad, a trailblazing enhancement in the world of ultrasound systems that is designed to simplify the user experience.
“During clinical evaluations performed in the US, we received enthusiastic customer feedback from those who used the Aixplorer MACH 30 and its touchpad,” says Kurt Kelln, chief business officer of sales for SuperSonic Imagine. “The SonicPad permits quick and intuitive control of all functions needed for a successful examination. It lets users improve their workflow while concentrating on the analysis of on-screen clinical information rather than which buttons to push for optimal image acquisition. The same enthusiastic reception was observed globally.”
The greater power of the Aixplorer MACH 30 means the innovative imaging modes it includes are now even faster and more efficient. B-mode image quality has improved, while other unique imaging modes—like Angio PL.U.S, offering unrivalled resolution for imaging microvascularization of lesions, and TriVu (B-mode / SWE / Color+), a new triplex mode combining three diagnostic data sets in a single examination—are enhanced by the new transducer range introduced by the Aixplorer MACH 30.
The Aixplorer MACH 30 ultrasound system applies an advanced ShearWave elastography (SWE) solution dubbed ShearWave PLUS. This technology built the reputation of SuperSonic Imagine and offers users real-time, reliable, quantitative, and reproducible 2D and 3D visualization and evaluation of tissue stiffness. Shortening acquisition speed and increasing examination depth for ever more reliable diagnostic data, this new generation of elastography technology accentuates the matchless capabilities developed by SuperSonic Imagine. Tissue stiffness has become a critical variable in the diagnosis of mammary and hepatic lesions and in the grading of breast and liver cancers. It offers greater diagnostic precision, more accurately distinguishing between benign and malign lesions and thus considerably reducing the number of false positives and unnecessary biopsies.
“The arrival of the Aixplorer MACH 30 opens new horizons for ultrasound,” concluded Jacques Souquet, SuperSonic Imagine founder and chief innovation officer. “We are eager to empower physicians with ever more powerful and effective tools for their daily professional activities.”
“The FDA 510(k) and CE mark are critical milestones in the launch of our exciting new system,” explains Michèle Lesieur, SuperSonic Imagine CEO and Chairwoman of its Board of Directors. “These approvals not only confirm that the Aixplorer MACH 30 is recognized for its quality, performance, and compliance with American and European regulatory standards, but it also clears the way for marketing of the system in the US, European member states, and many other countries around the world. We are confident this new innovative platform will offer clinical value which supports our growth ambitions in the Global Ultrasound Market.”
The new system represents another technological breakthrough from SuperSonic Imagine. Aixplorer MACH 30 offers the latest UltraFast imaging, enhancing a full range of innovative imaging modes developed by SuperSonic Imagine for superior diagnostic performance.
The greater performance, combined with an elegant and functional design and streamlined user experience of the Aixplorer MACH 30 will win over users around the world. It includes the SonicPad touchpad, a trailblazing enhancement in the world of ultrasound systems that is designed to simplify the user experience.
“During clinical evaluations performed in the US, we received enthusiastic customer feedback from those who used the Aixplorer MACH 30 and its touchpad,” says Kurt Kelln, chief business officer of sales for SuperSonic Imagine. “The SonicPad permits quick and intuitive control of all functions needed for a successful examination. It lets users improve their workflow while concentrating on the analysis of on-screen clinical information rather than which buttons to push for optimal image acquisition. The same enthusiastic reception was observed globally.”
The greater power of the Aixplorer MACH 30 means the innovative imaging modes it includes are now even faster and more efficient. B-mode image quality has improved, while other unique imaging modes—like Angio PL.U.S, offering unrivalled resolution for imaging microvascularization of lesions, and TriVu (B-mode / SWE / Color+), a new triplex mode combining three diagnostic data sets in a single examination—are enhanced by the new transducer range introduced by the Aixplorer MACH 30.
The Aixplorer MACH 30 ultrasound system applies an advanced ShearWave elastography (SWE) solution dubbed ShearWave PLUS. This technology built the reputation of SuperSonic Imagine and offers users real-time, reliable, quantitative, and reproducible 2D and 3D visualization and evaluation of tissue stiffness. Shortening acquisition speed and increasing examination depth for ever more reliable diagnostic data, this new generation of elastography technology accentuates the matchless capabilities developed by SuperSonic Imagine. Tissue stiffness has become a critical variable in the diagnosis of mammary and hepatic lesions and in the grading of breast and liver cancers. It offers greater diagnostic precision, more accurately distinguishing between benign and malign lesions and thus considerably reducing the number of false positives and unnecessary biopsies.
“The arrival of the Aixplorer MACH 30 opens new horizons for ultrasound,” concluded Jacques Souquet, SuperSonic Imagine founder and chief innovation officer. “We are eager to empower physicians with ever more powerful and effective tools for their daily professional activities.”