Globe Newswire08.30.18
Medtronic plc has announced new findings from the CRYO4PERSISTENT AF clinical trial demonstrating improved quality of life, reduced symptoms from abnormal heart rhythms, and low incidence of reinterventions and repeat ablation procedures. The study evaluated patients with symptomatic persistent atrial fibrillation (AF) treated with the Medtronic Arctic Front Advance(TM) Cryoballoon, and the results were presented at the 2018 European Society of Cardiology (ESC) Congress in Munich, Germany. The Arctic Front Advance Cryoablation System is not approved for treating persistent AF in the United States.
Patients treated with cryoballoon ablation experienced clinically meaningful changes in Quality of Life scores, with a 7.1 point average improvement in physical quality, and a 3.3 point average improvement in mental health quality component scores (using the SF-36 Short Form Health Survey, which is a validated tool routinely used in clinical research to assess health outcomes in clinical studies). Patients experienced a significant reduction in symptoms: While 92 percent of patients had symptoms such as dizziness, palpitations and fatigue prior to cryoablation, only 16 percent of patients had arrhythmia-related symptoms a year after treatment (P < 0.0001). The severity of symptoms also decreased after cryoballoon treatment, from 2.1 to 1.3 (p<0.01) using the European Heart Rhythm Association AF Symptom Score, and New York Heart Association (NYHA) class improved by one or more functional class in 47 percent of patients (from baseline to 12 months).
"These findings highlight the advantage of cryoballoon ablation in patients with persistent AF, demonstrating that patients have fewer symptoms after treatment and a significant improvement in their quality of life," said K.R. Julian Chun, M.D., Cardioangiological Center Bethanien, Agaplesion Markus Hospital, Frankfurt, Germany, and co-investigator of the study. "Paired with the study data showing a low incidence of reinterventions, it's clear to see the benefit for both patients and healthcare providers."
Although patients entering this study already had progressed to an advanced AF disease stage which traditionally has been more difficult to treat,1 the study found that only 17 of the 101 patients in the trial required a repeat ablation at the time of an arrhythmia recurrence. In addition, direct current cardioversion, a procedure to reset an abnormal heart rhythm back to normal, was only required for eight patients at the time of recurrence. Further, only three patients in sinus rhythm remained on antiarrhythmic medication at the completion of the study.
The primary CRYO4PERSISTENT AF study results, presented earlier this year and published this week in the Journal of the American College of Cardiology: Clinical Electrophysiology, demonstrated that 60.7 percent of patients were free from all atrial arrhythmias (adjudicated AF, atrial flutter or atrial tachyarrhythmias), at one year following a single PVI-only cryoballoon ablation procedure. The study findings also demonstrated short and predictable procedure times of 53 ± 22 minutes with the cryoballoon and a low complication rate of 4 percent.
"The CRYO4PERSISTENT AF study findings complement the growing body of clinical evidence that suggests cryoballoon ablation offers significant benefits to patients with AF even after the disease has progressed," said Rebecca Seidel, vice president and general manager of the AF Solutions business, part of the Cardiac and Vascular Group at Medtronic. "At Medtronic, we're focused on improving patient outcomes and quality of life, while offering greater efficiencies for physicians treating the growing number of AF patients today."
Medtronic is a world leader in the diagnosis, management and treatment of AF. AF is one of the most common heart rhythm disorders, affecting more than 33 million people worldwide.2 In Europe and the United States, AF affects approximately 10 and 5 million people, respectively. In both geographies, persistent AF represents approximately a quarter of all AF cases.3,4 Persistent AF occurs when the upper chambers of a patient's heart beat erratically for more than seven days and procedural intervention and/or drug therapy are required to stop the episode and restore normal sinus rhythm. Additionally, the risk of stroke and heart failure increases in patients with AF.5,6
CRYO4PERSISTENT AF is a prospective, single-arm, interventional, multi center, non-randomized clinical trial that evaluated 12-month clinical outcomes of cryoballoon ablation for isolating the pulmonary veins, without additional ablation in the left atrium, using the Medtronic Arctic Front Advance Cryoballoon System to treat patients with persistent AF. Eligible patients were defined as having documented symptomatic persistent AF at baseline lasting longer than seven days and up to 180 days. Prior to the procedure, enrolled patients were monitored to ensure they met the 100 percent persistent AF documentation criteria. Per protocol, a total of 101 patients were analyzed and followed for 12 months at 11 medical centers throughout Europe.
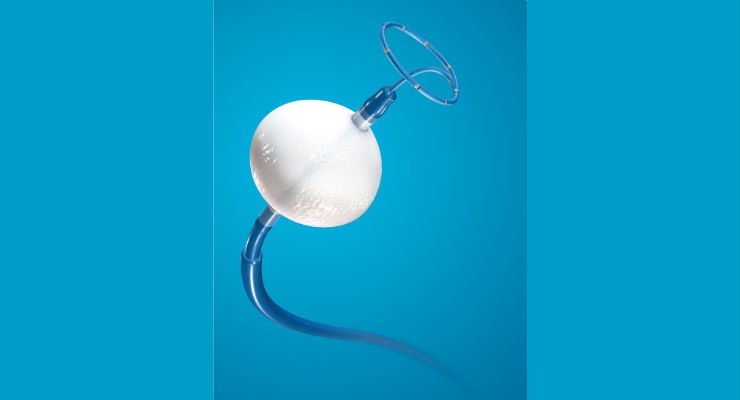
Cryoballoon ablation is used in a minimally invasive procedure to isolate the pulmonary veins, which are a source of erratic electrical signals that cause AF. The device uses cold energy rather than heat (radiofrequency, or RF, ablation) to create scar tissue and interrupt irregular electrical pathways in the heart. More than 400,000 patients in more than 60 countries worldwide have been treated with the cryoballoon. The Arctic Front Advance Cryoablation System is approved in Europe for the treatment of AF. In the United States, the Arctic Front Advance Cryoablation System is approved for the treatment of drug refractory, recurrent, symptomatic paroxysmal AF.
The 2016 ESC guidelines and the 2017 Heart Rhythm Society (HRS) Consensus Statement for the management of AF both acknowledge cryoablation therapy as a reasonable ablation energy for treating AF and recognize PVI as an effective and preferred treatment option for select patients with AF.
Medtronic plc, headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies. Medtronic employs more than 86,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries.
References
1. Ganesan A, Shipp N, Brooks A, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549.
2. Chugh S, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014; 129:837-847.
3. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clinical Epidemiology. 2014;6:213-220. doi:10.2147/CLEP.S47385.
4. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):e38-360
5. Fuster et al. Journal of the American College of Cardiology. 2006; 48:854-906.
6. ACC/AHA/ESC Guidelines for the Management of Patients with Atrial Fibrillation
Patients treated with cryoballoon ablation experienced clinically meaningful changes in Quality of Life scores, with a 7.1 point average improvement in physical quality, and a 3.3 point average improvement in mental health quality component scores (using the SF-36 Short Form Health Survey, which is a validated tool routinely used in clinical research to assess health outcomes in clinical studies). Patients experienced a significant reduction in symptoms: While 92 percent of patients had symptoms such as dizziness, palpitations and fatigue prior to cryoablation, only 16 percent of patients had arrhythmia-related symptoms a year after treatment (P < 0.0001). The severity of symptoms also decreased after cryoballoon treatment, from 2.1 to 1.3 (p<0.01) using the European Heart Rhythm Association AF Symptom Score, and New York Heart Association (NYHA) class improved by one or more functional class in 47 percent of patients (from baseline to 12 months).
"These findings highlight the advantage of cryoballoon ablation in patients with persistent AF, demonstrating that patients have fewer symptoms after treatment and a significant improvement in their quality of life," said K.R. Julian Chun, M.D., Cardioangiological Center Bethanien, Agaplesion Markus Hospital, Frankfurt, Germany, and co-investigator of the study. "Paired with the study data showing a low incidence of reinterventions, it's clear to see the benefit for both patients and healthcare providers."
Although patients entering this study already had progressed to an advanced AF disease stage which traditionally has been more difficult to treat,1 the study found that only 17 of the 101 patients in the trial required a repeat ablation at the time of an arrhythmia recurrence. In addition, direct current cardioversion, a procedure to reset an abnormal heart rhythm back to normal, was only required for eight patients at the time of recurrence. Further, only three patients in sinus rhythm remained on antiarrhythmic medication at the completion of the study.
The primary CRYO4PERSISTENT AF study results, presented earlier this year and published this week in the Journal of the American College of Cardiology: Clinical Electrophysiology, demonstrated that 60.7 percent of patients were free from all atrial arrhythmias (adjudicated AF, atrial flutter or atrial tachyarrhythmias), at one year following a single PVI-only cryoballoon ablation procedure. The study findings also demonstrated short and predictable procedure times of 53 ± 22 minutes with the cryoballoon and a low complication rate of 4 percent.
"The CRYO4PERSISTENT AF study findings complement the growing body of clinical evidence that suggests cryoballoon ablation offers significant benefits to patients with AF even after the disease has progressed," said Rebecca Seidel, vice president and general manager of the AF Solutions business, part of the Cardiac and Vascular Group at Medtronic. "At Medtronic, we're focused on improving patient outcomes and quality of life, while offering greater efficiencies for physicians treating the growing number of AF patients today."
Medtronic is a world leader in the diagnosis, management and treatment of AF. AF is one of the most common heart rhythm disorders, affecting more than 33 million people worldwide.2 In Europe and the United States, AF affects approximately 10 and 5 million people, respectively. In both geographies, persistent AF represents approximately a quarter of all AF cases.3,4 Persistent AF occurs when the upper chambers of a patient's heart beat erratically for more than seven days and procedural intervention and/or drug therapy are required to stop the episode and restore normal sinus rhythm. Additionally, the risk of stroke and heart failure increases in patients with AF.5,6
CRYO4PERSISTENT AF is a prospective, single-arm, interventional, multi center, non-randomized clinical trial that evaluated 12-month clinical outcomes of cryoballoon ablation for isolating the pulmonary veins, without additional ablation in the left atrium, using the Medtronic Arctic Front Advance Cryoballoon System to treat patients with persistent AF. Eligible patients were defined as having documented symptomatic persistent AF at baseline lasting longer than seven days and up to 180 days. Prior to the procedure, enrolled patients were monitored to ensure they met the 100 percent persistent AF documentation criteria. Per protocol, a total of 101 patients were analyzed and followed for 12 months at 11 medical centers throughout Europe.
Cryoballoon ablation is used in a minimally invasive procedure to isolate the pulmonary veins, which are a source of erratic electrical signals that cause AF. The device uses cold energy rather than heat (radiofrequency, or RF, ablation) to create scar tissue and interrupt irregular electrical pathways in the heart. More than 400,000 patients in more than 60 countries worldwide have been treated with the cryoballoon. The Arctic Front Advance Cryoablation System is approved in Europe for the treatment of AF. In the United States, the Arctic Front Advance Cryoablation System is approved for the treatment of drug refractory, recurrent, symptomatic paroxysmal AF.
The 2016 ESC guidelines and the 2017 Heart Rhythm Society (HRS) Consensus Statement for the management of AF both acknowledge cryoablation therapy as a reasonable ablation energy for treating AF and recognize PVI as an effective and preferred treatment option for select patients with AF.
Medtronic plc, headquartered in Dublin, Ireland, is among the world's largest medical technology, services and solutions companies. Medtronic employs more than 86,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries.
References
1. Ganesan A, Shipp N, Brooks A, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549.
2. Chugh S, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014; 129:837-847.
3. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clinical Epidemiology. 2014;6:213-220. doi:10.2147/CLEP.S47385.
4. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):e38-360
5. Fuster et al. Journal of the American College of Cardiology. 2006; 48:854-906.
6. ACC/AHA/ESC Guidelines for the Management of Patients with Atrial Fibrillation