Steven Mickelsen, MD, Chief Translational Science Officer, Acutus Medical 06.29.21
There is no denying that electroporation – or what is more commonly known as pulsed field ablation (PFA), DC ablation, and fulguration – has emerged as the hot topic among electrophysiologists in the preceding few years. Now, every major company selling ablation technology in the U.S. has an active PFA program with ongoing clinical trials in a race to determine the safety, efficacy, and efficiency of this emerging ablation strategy.
However, electroporation isn’t a new concept. Or a new technology for that matter. Not even remotely. In fact, its roots can be traced back to an 1867 publication in the British Medical Journal, which describes the use of electricity to treat tumors1. The use of fulguration or electric sparking to treat malignancies was practiced for many decades and studied until the 1930s. The foundation of electrophysiology as a cardiology subspecialty in the 1970s has electroporation as a part of its origin story. The field of interventional EP can be traced back to the spark of insight arising spontaneously from an experimental physiologist named J.W. Beazell, who was studying the impact of chronic pacing in the heart. He appeared to be the first person to use intravascular pulsed electricity to ablate heart tissue. His work at UCLA did not go unnoticed and led directly to preclinical investigations of the technique. By 1980, Melvin Scheinman and John Joseph Gallagher were already experimenting with direct current (DC) ablation techniques using a defibrillator to perform catheter ablation. And in 1982, they both published studies – Gallagher’s in the New England Journal of Medicine and Scheinman’s in the Journal of the American Medical Association – regarding the efficacy of DC ablation on the atrioventricular conduction system.
Thus, cardiac-directed pulsed field ablation was born. But not without a few concerns that needed to be addressed.
Firstly, there are innumerable things in the world that create electric fields – rain clouds, electric eels, human brains, the heart – but defibrillators create a spectacularly strong electric field. When concentrated into the tip of a catheter floating within the heart, the door is opened to a massive amount of electrolysis, and that can lead to a widespread disruption. This is what happened during many DC ablation procedures in the early 1980s – a dramatic consequence of electrolysis that produced what’s known as “flash arcing,” which can be explained using grade school physics – analogous to ice becoming water and water becoming steam. The hydrogen and oxygen from the electrolysis of water undergoes a phase transition as the electrical current is emitted. The result is almost like a miniature depth charge inside the heart, a situation that Buckaroo Banzai would have undoubtedly characterized as explosive.
By 1984 a number of innovators working in the field had identified the challenges of flash arcing and solved this problem by dramatically shortening the duration of electric discharge used to ablate. Now, pulses were measured in millionths of seconds – this was not long enough to generate the spectacular flash arcing observed with defibrillators. By 1986 the first recognizable electroporation ablation system was commercially available when the National Heart Hospital’s low energy pulse generator entered the stage. This system reduced the total energy required for an ablation by an order of magnitude and practically eliminated flash arcing altogether. Research on cardiac electroporation continued to evolve with the development of specialized ablation catheters that distributed current over larger electrodes and shapes that reduced edge effects.
Secondly, there were additional challenges to the adoption of DC ablation in clinical application. Arguably, the need for deep sedation was chief among these. After all, a kilovolt pulse was something that could make a patient jump to attention. By the mid-1980s there was widespread misconception that the mechanism of tissue injury was barotrauma resulting from the tiny explosion and not cell injury through electroporation. This false understanding led many electrophysiologists to believe that DC ablation was not very precise or targeted, or the safest way to perform ablation—a logical conclusion given the startling characteristics of flash arcing and physiological optics of defibrillation. This conclusion, however, was not supported by the preclinical research showing predictable lesion volumes, remarkably fast wound healing, and tissue selectivity. Nor was it supported by clinical research showing that the safety of performing DC ablation was no different from radio frequency (RF) ablation. That said, RF catheter ablation became the predominant technology used in arrhythmia treatment by the 1990s while DC ablation all but disappeared.
Radiofrequency (RF) catheter ablation emerged late in 1986 as an innovative approach to cardiac ablation, demonstrating real value because it could be used in lightly sedated patients. This single factor, more than any other, provided huge advantages for procedural workflow in the then-burgeoning field of interventional electrophysiology. However, the next 30 years of research, development, and clinical research would demonstrate that perhaps cooking tissue with RF energy was not as precise or as targeted, or necessarily the safest way to perform ablation.
My own work with electroporation began in earnest around 2011 at the University of Iowa, where I started to experiment with new approaches to atrial fibrillation (AF) ablation. These efforts led me to invent an epicardial catheter-based procedure taking advantage of PFA. The time, efficiency, tissue selectivity, and ease of delivery made PFA the clear choice. The University filed a patent on my work, and I started a company to license my own technology back from the university. At the time Melvin Scheinman – the first physician to perform catheter ablation in a human – thought my PFA approach was “eminently doable” and a number of preeminent electrophysiologists, including Andrea Natale and Fred Kusumoto, supported my application for a SBIR grant to develop the technology. The resulting company was originally called Iowa Approach while I focused on preclinical R&D, fund raising, and recruiting the leadership team. Iowa Approach became an important part of the story of electroporation when the esteemed electrophysiologist Vivek Reddy and his colleagues reported results from the first human trial to use PFA since the 1980s. This publication ignited a shift in the paradigms we see unfolding today. Shortly after, we changed the name of the company to Farapulse and so began the full-blown reemergence of PFA in clinical cardiac electrophysiology.
When we consider electroporation’s recent Phoenix-like return to mainstream cardiac electrophysiology, there are a few primary advantages that must be discussed. These advantages are best highlighted in the application for catheter ablation of AF.
Catheter ablation to treat AF largely emerged from the disruptive work of two highly esteemed electrophysiologists, Michel Haïssaguerre and Pier Jaïs, in the mid-1990s. At that time, the tools of the trade were RF ablation catheters, fluoroscopic navigation, and skilled interpretation of intracardiac electrograms. From this springboard of insight, the global understanding, tools, and technologies improved over intervening decades, leading to the widespread practice of pulmonary vein isolation (PVI) as the cornerstone approach to AF ablation. Yet, as gathering clinical research established the value of AF ablation, shortcomings also became more visible, in particular the risk to the phrenic nerve, esophagus, and brain.
Thermal ablation and RF ablation are well-understood, effective, and highly refined. However, all soft tissues in the body are susceptible to temperature-mediated ablation – hot or cold – more or less at the same temperature point. This fact makes it challenging to ablate targeted tissue without also ablating nearby tissues. This is where PFA differs significantly. The mode of PFA injury is predominantly interference with critical metabolic function, and tissue susceptibility to this type of insult differs widely. Cardiac myocytes happen to be exquisitely sensitive to PFA in comparison to almost every other tissue type found in the body. This helps explain observations that show PFA lesions retaining intracellular collagen structure and blood supply. Within a PFA lesion, one can observe blood vessels, nerves, and endothelium untouched while the targeted cardiac myocytes are completely ablated. The practical consequence is rapid tissue healing – typically resolving in two weeks. This is ideal for applications such as AF ablation, where the sensitive atrial tissue can be targeted with a practical safety margin for nearby “less sensitive” structures such as the esophagus and phrenic nerve, a feat that is simply not possible with high temperature ablation.
Contemporary PFA technologies under development utilize short bursts of high voltage microsecond or nanosecond pulses delivered to target tissue through one or more electrodes. Several strategies are starting to converge as the principal approaches in the field. First, there are what we call “single shot” PVI devices. This type of technology follows in the workflow with a cryoballoon where a tool is positioned in the ostium of a target vein and ablation is performed all at once. Examples of this type of technology are under clinical investigation from companies such as Farapulse, Medtronic, Abbott, and Biosense Webster.
The second strategy involves tools that follow the more traditional “point-by-point” approach familiar to the RF ablation catheter workflow. Here, linear lesions are created by ablating sequentially to either encircle a target vein or create any variety of lesions that are possible with a traditional RF catheter. Examples of this technology are being developed by Acutus Medical, Afera, and Farapulse. Compared to the 1980s, modern PFA waveforms appear to be favoring biphasic pulses in order to reduce the jump associated with excitation. This is an important consideration if PFA technology is going to be used in lightly or moderately sedated patients. However, one of the engineering challenges with biphasic waveforms comes from efficiency. They are less effective than monophasic pulses at creating lesions for a given amount of energy used, and the rapid oscillation leads to more heating, similar to RF, thus negating some of the benefits of using PFA. When titrated correctly over time or distributed across many electrodes, heating can be managed. The use of monophasic waveforms is advantageous for time and temperature efficiency. In clinical procedures where patients are deeply sedated, or under general anesthesia, the jump/twitch is manageable and tolerated.
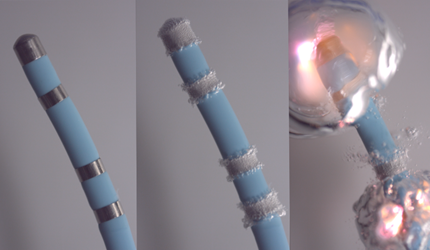
This figure illustrates the sequence of a flash arching event from a defibrillator attached to a catheter. In the second pane, bubbles result from a strong electric field producing electrolysis (Panel 2). At some point, the gas becomes a plasma, and the ensuing pressure wave pushes fluid away from the electrode (Panel 3).
As the field of cardiac PFA evolves, there are likely to be a wide range of treatment strategies that can be optimized for each intended use. What is important to understand is that the variables for PFA waveform development are substantially more complex than traditional RF applications. These include electrode geometry, peak voltage, pulse duration, pulse repetition, pulse repetition pattern, and/or phase of pulse, just to name a few of the major determinants. Understanding the target tissue physiology also becomes critical to success as tissue cell size, shape, orientation in the field, cell membrane constitution, cellular energetics, PH, extracellular environment, and temperature can all impact the results. The underlying complexity and variety of dosing strategies makes it almost impossible to directly compare results from different catheter-waveform treatment strategies. That said, PFA can be equally effective despite using very different waveform structures and catheter configurations.
But the key to PFA’s true value may not actually lie in the technology itself but in how the technology lends itself to more individualized treatment strategies and integration into contemporary 3D mapping applications. This is because PFA lesion volume and contours follow well-defined and predictable electromagnetic rules, whereas thermal ablation follows dynamics of heat transfer with significant increases in complexity. Because the PFA field is predictable, doctors may be able to plan the procedures with much greater confidence.
Since joining Acutus Medical as the Chief Translational Science Officer, I have focused on merging the technical possibilities with the clinical needs of my field. What was once almost science fiction is now eminently doable with the tools that Acutus is developing. We can map irregular heart rhythms like atrial fibrillation without ever touching the inside of the heart. This is not possible with contact mapping systems from Biosense Webster, Abbott, or Boston Scientific. The AcQMap system opens the door to expanding atrial fibrillation treatment strategies beyond PVI by identifying potential targets that physicians are just now beginning to assess. Recently published ablation strategies like “Core-to-Boundary” are showing great promises in the treatment of persistent AF patients.
A number of ongoing investigator-initiated studies are now pointing this powerful mapping technology at the constellation of previously unseen patterns and functional characteristics of AF. These are exciting times, like Galileo pointing his telescope to the havens for the first time. I can see a future where we can quickly create a 3D electroanatomic map of any arrhythmia and plan treatment strategies based on individualized knowledge. Here is where integration with PFA technologies may have a significant advantage in delivering tailored treatment with dramatically improved reliability and workflow. I do not believe I am alone in this proposition given the enormous financial resources currently being poured into developing PFA from every corner of the EP technology space.
It’s ironic that the original pioneers of catheter ablation may have stumbled onto the right tool from the very beginning. Like Dorothy in The Wizard of Oz walking the yellow brick road to get home in her sparkling ruby slippers, we may have had the ability to go home the entire time.
Reference:
1Collis MH. “On the Treatment of Tumors by Electricity.” Br Med J. 1867 Dec 7;2(362):521-2
Dr. Steven Mickelsen currently serves as the chief translational science officer of Acutus Medical, guiding the innovation of the company’s access, diagnostics and therapy product portfolio, and brings more than a decade of experience in the development of breakthrough therapeutic medical devices. A board-certified electrophysiologist, Mickelsen spent almost ten years practicing at the University of Iowa Hospitals and Clinics in Iowa City, where he focused on complex ablation procedures and served as an assistant professor of Cardiovascular Medicine and Internal Medicine. In 2012, utilizing a pulsed electric field system that he invented, Mickelsen founded the company Farapulse to pursue more effective treatments for atrial fibrillation, a condition that affects as many as six million people nationwide. Mickelsen received his medical degree from the University of New Mexico School of Medicine and completed his internal medicine residency and clinical research at the Mayo Clinic. He also completed fellowships in Cardiology and Cardiac Electrophysiology at the University of Iowa Hospitals and Clinics and was a Howard Hughes research scholar at the National Institutes of Health.
However, electroporation isn’t a new concept. Or a new technology for that matter. Not even remotely. In fact, its roots can be traced back to an 1867 publication in the British Medical Journal, which describes the use of electricity to treat tumors1. The use of fulguration or electric sparking to treat malignancies was practiced for many decades and studied until the 1930s. The foundation of electrophysiology as a cardiology subspecialty in the 1970s has electroporation as a part of its origin story. The field of interventional EP can be traced back to the spark of insight arising spontaneously from an experimental physiologist named J.W. Beazell, who was studying the impact of chronic pacing in the heart. He appeared to be the first person to use intravascular pulsed electricity to ablate heart tissue. His work at UCLA did not go unnoticed and led directly to preclinical investigations of the technique. By 1980, Melvin Scheinman and John Joseph Gallagher were already experimenting with direct current (DC) ablation techniques using a defibrillator to perform catheter ablation. And in 1982, they both published studies – Gallagher’s in the New England Journal of Medicine and Scheinman’s in the Journal of the American Medical Association – regarding the efficacy of DC ablation on the atrioventricular conduction system.
Thus, cardiac-directed pulsed field ablation was born. But not without a few concerns that needed to be addressed.
Firstly, there are innumerable things in the world that create electric fields – rain clouds, electric eels, human brains, the heart – but defibrillators create a spectacularly strong electric field. When concentrated into the tip of a catheter floating within the heart, the door is opened to a massive amount of electrolysis, and that can lead to a widespread disruption. This is what happened during many DC ablation procedures in the early 1980s – a dramatic consequence of electrolysis that produced what’s known as “flash arcing,” which can be explained using grade school physics – analogous to ice becoming water and water becoming steam. The hydrogen and oxygen from the electrolysis of water undergoes a phase transition as the electrical current is emitted. The result is almost like a miniature depth charge inside the heart, a situation that Buckaroo Banzai would have undoubtedly characterized as explosive.
By 1984 a number of innovators working in the field had identified the challenges of flash arcing and solved this problem by dramatically shortening the duration of electric discharge used to ablate. Now, pulses were measured in millionths of seconds – this was not long enough to generate the spectacular flash arcing observed with defibrillators. By 1986 the first recognizable electroporation ablation system was commercially available when the National Heart Hospital’s low energy pulse generator entered the stage. This system reduced the total energy required for an ablation by an order of magnitude and practically eliminated flash arcing altogether. Research on cardiac electroporation continued to evolve with the development of specialized ablation catheters that distributed current over larger electrodes and shapes that reduced edge effects.
Secondly, there were additional challenges to the adoption of DC ablation in clinical application. Arguably, the need for deep sedation was chief among these. After all, a kilovolt pulse was something that could make a patient jump to attention. By the mid-1980s there was widespread misconception that the mechanism of tissue injury was barotrauma resulting from the tiny explosion and not cell injury through electroporation. This false understanding led many electrophysiologists to believe that DC ablation was not very precise or targeted, or the safest way to perform ablation—a logical conclusion given the startling characteristics of flash arcing and physiological optics of defibrillation. This conclusion, however, was not supported by the preclinical research showing predictable lesion volumes, remarkably fast wound healing, and tissue selectivity. Nor was it supported by clinical research showing that the safety of performing DC ablation was no different from radio frequency (RF) ablation. That said, RF catheter ablation became the predominant technology used in arrhythmia treatment by the 1990s while DC ablation all but disappeared.
Radiofrequency (RF) catheter ablation emerged late in 1986 as an innovative approach to cardiac ablation, demonstrating real value because it could be used in lightly sedated patients. This single factor, more than any other, provided huge advantages for procedural workflow in the then-burgeoning field of interventional electrophysiology. However, the next 30 years of research, development, and clinical research would demonstrate that perhaps cooking tissue with RF energy was not as precise or as targeted, or necessarily the safest way to perform ablation.
My own work with electroporation began in earnest around 2011 at the University of Iowa, where I started to experiment with new approaches to atrial fibrillation (AF) ablation. These efforts led me to invent an epicardial catheter-based procedure taking advantage of PFA. The time, efficiency, tissue selectivity, and ease of delivery made PFA the clear choice. The University filed a patent on my work, and I started a company to license my own technology back from the university. At the time Melvin Scheinman – the first physician to perform catheter ablation in a human – thought my PFA approach was “eminently doable” and a number of preeminent electrophysiologists, including Andrea Natale and Fred Kusumoto, supported my application for a SBIR grant to develop the technology. The resulting company was originally called Iowa Approach while I focused on preclinical R&D, fund raising, and recruiting the leadership team. Iowa Approach became an important part of the story of electroporation when the esteemed electrophysiologist Vivek Reddy and his colleagues reported results from the first human trial to use PFA since the 1980s. This publication ignited a shift in the paradigms we see unfolding today. Shortly after, we changed the name of the company to Farapulse and so began the full-blown reemergence of PFA in clinical cardiac electrophysiology.
When we consider electroporation’s recent Phoenix-like return to mainstream cardiac electrophysiology, there are a few primary advantages that must be discussed. These advantages are best highlighted in the application for catheter ablation of AF.
Catheter ablation to treat AF largely emerged from the disruptive work of two highly esteemed electrophysiologists, Michel Haïssaguerre and Pier Jaïs, in the mid-1990s. At that time, the tools of the trade were RF ablation catheters, fluoroscopic navigation, and skilled interpretation of intracardiac electrograms. From this springboard of insight, the global understanding, tools, and technologies improved over intervening decades, leading to the widespread practice of pulmonary vein isolation (PVI) as the cornerstone approach to AF ablation. Yet, as gathering clinical research established the value of AF ablation, shortcomings also became more visible, in particular the risk to the phrenic nerve, esophagus, and brain.
Thermal ablation and RF ablation are well-understood, effective, and highly refined. However, all soft tissues in the body are susceptible to temperature-mediated ablation – hot or cold – more or less at the same temperature point. This fact makes it challenging to ablate targeted tissue without also ablating nearby tissues. This is where PFA differs significantly. The mode of PFA injury is predominantly interference with critical metabolic function, and tissue susceptibility to this type of insult differs widely. Cardiac myocytes happen to be exquisitely sensitive to PFA in comparison to almost every other tissue type found in the body. This helps explain observations that show PFA lesions retaining intracellular collagen structure and blood supply. Within a PFA lesion, one can observe blood vessels, nerves, and endothelium untouched while the targeted cardiac myocytes are completely ablated. The practical consequence is rapid tissue healing – typically resolving in two weeks. This is ideal for applications such as AF ablation, where the sensitive atrial tissue can be targeted with a practical safety margin for nearby “less sensitive” structures such as the esophagus and phrenic nerve, a feat that is simply not possible with high temperature ablation.
Contemporary PFA technologies under development utilize short bursts of high voltage microsecond or nanosecond pulses delivered to target tissue through one or more electrodes. Several strategies are starting to converge as the principal approaches in the field. First, there are what we call “single shot” PVI devices. This type of technology follows in the workflow with a cryoballoon where a tool is positioned in the ostium of a target vein and ablation is performed all at once. Examples of this type of technology are under clinical investigation from companies such as Farapulse, Medtronic, Abbott, and Biosense Webster.
The second strategy involves tools that follow the more traditional “point-by-point” approach familiar to the RF ablation catheter workflow. Here, linear lesions are created by ablating sequentially to either encircle a target vein or create any variety of lesions that are possible with a traditional RF catheter. Examples of this technology are being developed by Acutus Medical, Afera, and Farapulse. Compared to the 1980s, modern PFA waveforms appear to be favoring biphasic pulses in order to reduce the jump associated with excitation. This is an important consideration if PFA technology is going to be used in lightly or moderately sedated patients. However, one of the engineering challenges with biphasic waveforms comes from efficiency. They are less effective than monophasic pulses at creating lesions for a given amount of energy used, and the rapid oscillation leads to more heating, similar to RF, thus negating some of the benefits of using PFA. When titrated correctly over time or distributed across many electrodes, heating can be managed. The use of monophasic waveforms is advantageous for time and temperature efficiency. In clinical procedures where patients are deeply sedated, or under general anesthesia, the jump/twitch is manageable and tolerated.

This figure illustrates the sequence of a flash arching event from a defibrillator attached to a catheter. In the second pane, bubbles result from a strong electric field producing electrolysis (Panel 2). At some point, the gas becomes a plasma, and the ensuing pressure wave pushes fluid away from the electrode (Panel 3).
As the field of cardiac PFA evolves, there are likely to be a wide range of treatment strategies that can be optimized for each intended use. What is important to understand is that the variables for PFA waveform development are substantially more complex than traditional RF applications. These include electrode geometry, peak voltage, pulse duration, pulse repetition, pulse repetition pattern, and/or phase of pulse, just to name a few of the major determinants. Understanding the target tissue physiology also becomes critical to success as tissue cell size, shape, orientation in the field, cell membrane constitution, cellular energetics, PH, extracellular environment, and temperature can all impact the results. The underlying complexity and variety of dosing strategies makes it almost impossible to directly compare results from different catheter-waveform treatment strategies. That said, PFA can be equally effective despite using very different waveform structures and catheter configurations.
But the key to PFA’s true value may not actually lie in the technology itself but in how the technology lends itself to more individualized treatment strategies and integration into contemporary 3D mapping applications. This is because PFA lesion volume and contours follow well-defined and predictable electromagnetic rules, whereas thermal ablation follows dynamics of heat transfer with significant increases in complexity. Because the PFA field is predictable, doctors may be able to plan the procedures with much greater confidence.
Since joining Acutus Medical as the Chief Translational Science Officer, I have focused on merging the technical possibilities with the clinical needs of my field. What was once almost science fiction is now eminently doable with the tools that Acutus is developing. We can map irregular heart rhythms like atrial fibrillation without ever touching the inside of the heart. This is not possible with contact mapping systems from Biosense Webster, Abbott, or Boston Scientific. The AcQMap system opens the door to expanding atrial fibrillation treatment strategies beyond PVI by identifying potential targets that physicians are just now beginning to assess. Recently published ablation strategies like “Core-to-Boundary” are showing great promises in the treatment of persistent AF patients.
A number of ongoing investigator-initiated studies are now pointing this powerful mapping technology at the constellation of previously unseen patterns and functional characteristics of AF. These are exciting times, like Galileo pointing his telescope to the havens for the first time. I can see a future where we can quickly create a 3D electroanatomic map of any arrhythmia and plan treatment strategies based on individualized knowledge. Here is where integration with PFA technologies may have a significant advantage in delivering tailored treatment with dramatically improved reliability and workflow. I do not believe I am alone in this proposition given the enormous financial resources currently being poured into developing PFA from every corner of the EP technology space.
It’s ironic that the original pioneers of catheter ablation may have stumbled onto the right tool from the very beginning. Like Dorothy in The Wizard of Oz walking the yellow brick road to get home in her sparkling ruby slippers, we may have had the ability to go home the entire time.
Reference:
1Collis MH. “On the Treatment of Tumors by Electricity.” Br Med J. 1867 Dec 7;2(362):521-2
Dr. Steven Mickelsen currently serves as the chief translational science officer of Acutus Medical, guiding the innovation of the company’s access, diagnostics and therapy product portfolio, and brings more than a decade of experience in the development of breakthrough therapeutic medical devices. A board-certified electrophysiologist, Mickelsen spent almost ten years practicing at the University of Iowa Hospitals and Clinics in Iowa City, where he focused on complex ablation procedures and served as an assistant professor of Cardiovascular Medicine and Internal Medicine. In 2012, utilizing a pulsed electric field system that he invented, Mickelsen founded the company Farapulse to pursue more effective treatments for atrial fibrillation, a condition that affects as many as six million people nationwide. Mickelsen received his medical degree from the University of New Mexico School of Medicine and completed his internal medicine residency and clinical research at the Mayo Clinic. He also completed fellowships in Cardiology and Cardiac Electrophysiology at the University of Iowa Hospitals and Clinics and was a Howard Hughes research scholar at the National Institutes of Health.





















