Business Wire05.30.18
PAVmed Inc., a multi-product medical device company, has signed a letter of intent with Case Western Reserve University to commercialize its EsoCheck technology.
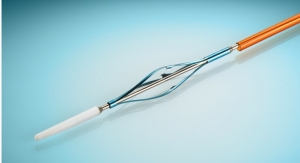
EsoCheck combines a non-invasive, cell-sampling device with highly accurate DNA biomarkers to detect Barrett’s Esophagus, the primary precursor of esophageal cancer. In a simple five-minute office-based test, the patient swallows a vitamin pill-sized capsule containing a small inflatable balloon attached to a thin catheter, which swabs the target area for cells as the catheter is withdrawn. The sample is then tested for the presence of DNA biomarkers recently shown to be highly accurate in detecting Barrett’s Esophagus.
“We are very excited about the opportunity to work with the world-renowned Case Western Reserve faculty to commercialize this revolutionary technology,” said PAVmed Chairman and CEO Lishan Aklog, M.D. “Based on the results of a multicenter clinical study, recently published in a seminal article in Science Translational Medicine, we believe EsoCheck has the potential to replicate the impact of Pap screening for cervical cancer and colonoscopy screening for colon cancer in preventing deaths from esophageal cancer. Once consummated, this partnership will fulfill one of PAVmed’s central goals: to provide innovators and academic medical centers with a rapid, capital-efficient and streamlined pathway to commercialization of high-impact innovative products. By leveraging the substantial clinical work already completed by the team and interim results from an ongoing multicenter National Institutes of Health trial, we believe we can accelerate EsoCheck’s commercialization path and target the first quarter of 2019 for the launch of the first commercial product.”
The incidence of esophageal adenocarcinoma (EAC), the most common cancer of the esophagus (the pipe through which food passes to the stomach), has quadrupled over the past 30 years. Its prognosis, however, remains dismal, with less than 20 percent of patients surviving five years.
“The Case Western Reserve/University Hospitals team looks forward to consummating this partnership. We have been impressed by the PAVmed team’s track record of getting innovative products to market using a lean and efficient business model,” said Daniel I. Simon, M.D., president and chief academic officer of University Hospitals Cleveland Medical Center and professor of medicine at Case Western Reserve University School of Medicine. “Their unique and creative partnership model, which fully aligns our scientific and humanitarian interests with the commercial enterprise, is highly attractive to academic medical centers seeking to bring cutting-edge, life-saving technologies to patients.”
The primary cause of EAC cancer is Gastroesophageal Reflux Disease (GERD), commonly known as chronic heartburn or acid reflux. GERD, where stomach acid refluxes into the esophagus, affects 20 percent to 40 percent of Western adult populations, according to published epidemiological data.
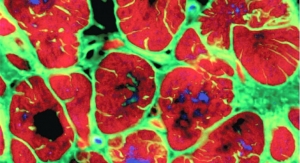
The repeated exposure of the esophagus to acid can lead to pre-cancerous changes in its lining, called Barrett’s Esophagus. Nearly all patients diagnosed with EAC cancer have evidence of previously undetected Barrett’s Esophagus. If detected before EAC cancer develops, Barrett’s Esophagus can be successfully treated, usually with non-surgical approaches.
“Our goal is to save lives through early detection,” said EsoCheck co-inventor Amitabh Chak, M.D., professor of medicine at Case Western University School of Medicine, gastroenterologist at UH Digestive Health Institute, and director of the Advanced Technology & Innovation Center of Excellence at University Hospitals Division of Gastroenterology and editorial board chair of Gastrointestinal Endoscopy, the pre-eminent journal in the field. “Heartburn symptoms, commonly seen in patients with acid reflux with or without Barrett’s, can easily be treated by over-the counter medications, while endoscopy, the standard diagnostic test, is expensive, invasive and requires sedation. As a result, wide screening for Barrett’s is not practical or cost-effective. We developed EsoCheck to provide the estimated 50 million at-risk patients a non-invasive, less costly test to detect Barrett’s and treat it before it turns deadly.”
The EsoCheck kit includes a sampling device consisting of a vitamin pill-sized, silicone-covered capsule containing a small deflated balloon attached to a thin silicone catheter. The patient swallows the capsule and, once it reaches the stomach, the balloon is inflated with air. As the balloon is pulled back, it swabs the lower esophagus for cells. After a specified distance, the balloon is deflated so, in contrast to other esophageal sampling devices, the sample of cells is protected from dilution or contamination as it passes back through the upper esophagus and mouth.
DNA is extracted from the cells and tested for a panel of DNA biomarkers developed by the laboratory of EsoCheck co-inventor Sanford Markowitz, M.D., Ph.D., the Ingalls Professor of Cancer Genetics medical oncologist at University Hospitals Seidman Cancer Center, NCI Outstanding Investigator Awardee, and head of the NIH-Case GI Cancers Program of Research Excellence (GI SPORE) and GI cancer genetics program at the Case Comprehensive Cancer Center.
The Science Translational Medicine paper showed that DNA methylation of the VIM and CCNA1 genes is diagnostic of Barrett’s Esophagus and that EsoCheck, which combines the proprietary balloon sampling device with these biomarkers, was over 90 percent accurate at identifying patients without Barrett’s Esophagus. The third EsoCheck co-inventor, Joseph Willis, M.D., professor of pathology and pathology vice-chair for clinical affairs, at University Hospitals Cleveland Medical Center, is leading an ongoing NIH-supported effort to create a CLIA-certified VIM/CCNA1 DNA methylation test suitable for commercialization.
Under terms outlined in the letter of intent, a PAVmed subsidiary and Case Western Reserve University propose to enter into a definitive license agreement in which the university will grant the subsidiary an exclusive worldwide license of the intellectual property rights for both the balloon sampling device and DNA biomarkers of EsoCheck.
In return, Case Western Reserve will receive a non-cash license fee in the form of a minority equity interest in the subsidiary, with PAVmed retaining a supermajority equity interest. The agreement will be subject to certain regulatory and commercialization milestones, with the university receiving royalties based on revenue and a specified portion of any additional proceeds.
The subsidiary will operate as a separate entity, raise its own capital and recruit a separate management team when deemed appropriate.
The letter of intent does not constitute a binding contract and the proposed license will not be granted unless and until a definitive License Agreement has been executed by both parties.
PAVmed Inc. is a highly differentiated, multiproduct medical device company employing a business model designed to advance innovative products to commercialization much more rapidly and with significantly less capital than the typical medical device company. This proprietary model enables PAVmed to pursue an expanding pipeline strategy with a view to enhancing and accelerating value creation. PAVmed’s pipeline of products address unmet clinical needs encompassing a broad spectrum of clinical areas with attractive regulatory pathways and market opportunities. Its three lead products provide groundbreaking approaches to carpal tunnel syndrome (CarpX), vascular access (PortIO) and pediatric ear infections (DisappEAR). The company is also developing products in other areas, such as medical infusions and tissue ablation, while seeking to further expand its pipeline through engagements with clinician innovators and leading academic medical centers.
EsoCheck combines a non-invasive, cell-sampling device with highly accurate DNA biomarkers to detect Barrett’s Esophagus, the primary precursor of esophageal cancer. In a simple five-minute office-based test, the patient swallows a vitamin pill-sized capsule containing a small inflatable balloon attached to a thin catheter, which swabs the target area for cells as the catheter is withdrawn. The sample is then tested for the presence of DNA biomarkers recently shown to be highly accurate in detecting Barrett’s Esophagus.
“We are very excited about the opportunity to work with the world-renowned Case Western Reserve faculty to commercialize this revolutionary technology,” said PAVmed Chairman and CEO Lishan Aklog, M.D. “Based on the results of a multicenter clinical study, recently published in a seminal article in Science Translational Medicine, we believe EsoCheck has the potential to replicate the impact of Pap screening for cervical cancer and colonoscopy screening for colon cancer in preventing deaths from esophageal cancer. Once consummated, this partnership will fulfill one of PAVmed’s central goals: to provide innovators and academic medical centers with a rapid, capital-efficient and streamlined pathway to commercialization of high-impact innovative products. By leveraging the substantial clinical work already completed by the team and interim results from an ongoing multicenter National Institutes of Health trial, we believe we can accelerate EsoCheck’s commercialization path and target the first quarter of 2019 for the launch of the first commercial product.”
The incidence of esophageal adenocarcinoma (EAC), the most common cancer of the esophagus (the pipe through which food passes to the stomach), has quadrupled over the past 30 years. Its prognosis, however, remains dismal, with less than 20 percent of patients surviving five years.
“The Case Western Reserve/University Hospitals team looks forward to consummating this partnership. We have been impressed by the PAVmed team’s track record of getting innovative products to market using a lean and efficient business model,” said Daniel I. Simon, M.D., president and chief academic officer of University Hospitals Cleveland Medical Center and professor of medicine at Case Western Reserve University School of Medicine. “Their unique and creative partnership model, which fully aligns our scientific and humanitarian interests with the commercial enterprise, is highly attractive to academic medical centers seeking to bring cutting-edge, life-saving technologies to patients.”
The primary cause of EAC cancer is Gastroesophageal Reflux Disease (GERD), commonly known as chronic heartburn or acid reflux. GERD, where stomach acid refluxes into the esophagus, affects 20 percent to 40 percent of Western adult populations, according to published epidemiological data.
The repeated exposure of the esophagus to acid can lead to pre-cancerous changes in its lining, called Barrett’s Esophagus. Nearly all patients diagnosed with EAC cancer have evidence of previously undetected Barrett’s Esophagus. If detected before EAC cancer develops, Barrett’s Esophagus can be successfully treated, usually with non-surgical approaches.
“Our goal is to save lives through early detection,” said EsoCheck co-inventor Amitabh Chak, M.D., professor of medicine at Case Western University School of Medicine, gastroenterologist at UH Digestive Health Institute, and director of the Advanced Technology & Innovation Center of Excellence at University Hospitals Division of Gastroenterology and editorial board chair of Gastrointestinal Endoscopy, the pre-eminent journal in the field. “Heartburn symptoms, commonly seen in patients with acid reflux with or without Barrett’s, can easily be treated by over-the counter medications, while endoscopy, the standard diagnostic test, is expensive, invasive and requires sedation. As a result, wide screening for Barrett’s is not practical or cost-effective. We developed EsoCheck to provide the estimated 50 million at-risk patients a non-invasive, less costly test to detect Barrett’s and treat it before it turns deadly.”
The EsoCheck kit includes a sampling device consisting of a vitamin pill-sized, silicone-covered capsule containing a small deflated balloon attached to a thin silicone catheter. The patient swallows the capsule and, once it reaches the stomach, the balloon is inflated with air. As the balloon is pulled back, it swabs the lower esophagus for cells. After a specified distance, the balloon is deflated so, in contrast to other esophageal sampling devices, the sample of cells is protected from dilution or contamination as it passes back through the upper esophagus and mouth.
DNA is extracted from the cells and tested for a panel of DNA biomarkers developed by the laboratory of EsoCheck co-inventor Sanford Markowitz, M.D., Ph.D., the Ingalls Professor of Cancer Genetics medical oncologist at University Hospitals Seidman Cancer Center, NCI Outstanding Investigator Awardee, and head of the NIH-Case GI Cancers Program of Research Excellence (GI SPORE) and GI cancer genetics program at the Case Comprehensive Cancer Center.
The Science Translational Medicine paper showed that DNA methylation of the VIM and CCNA1 genes is diagnostic of Barrett’s Esophagus and that EsoCheck, which combines the proprietary balloon sampling device with these biomarkers, was over 90 percent accurate at identifying patients without Barrett’s Esophagus. The third EsoCheck co-inventor, Joseph Willis, M.D., professor of pathology and pathology vice-chair for clinical affairs, at University Hospitals Cleveland Medical Center, is leading an ongoing NIH-supported effort to create a CLIA-certified VIM/CCNA1 DNA methylation test suitable for commercialization.
Under terms outlined in the letter of intent, a PAVmed subsidiary and Case Western Reserve University propose to enter into a definitive license agreement in which the university will grant the subsidiary an exclusive worldwide license of the intellectual property rights for both the balloon sampling device and DNA biomarkers of EsoCheck.
In return, Case Western Reserve will receive a non-cash license fee in the form of a minority equity interest in the subsidiary, with PAVmed retaining a supermajority equity interest. The agreement will be subject to certain regulatory and commercialization milestones, with the university receiving royalties based on revenue and a specified portion of any additional proceeds.
The subsidiary will operate as a separate entity, raise its own capital and recruit a separate management team when deemed appropriate.
The letter of intent does not constitute a binding contract and the proposed license will not be granted unless and until a definitive License Agreement has been executed by both parties.
PAVmed Inc. is a highly differentiated, multiproduct medical device company employing a business model designed to advance innovative products to commercialization much more rapidly and with significantly less capital than the typical medical device company. This proprietary model enables PAVmed to pursue an expanding pipeline strategy with a view to enhancing and accelerating value creation. PAVmed’s pipeline of products address unmet clinical needs encompassing a broad spectrum of clinical areas with attractive regulatory pathways and market opportunities. Its three lead products provide groundbreaking approaches to carpal tunnel syndrome (CarpX), vascular access (PortIO) and pediatric ear infections (DisappEAR). The company is also developing products in other areas, such as medical infusions and tissue ablation, while seeking to further expand its pipeline through engagements with clinician innovators and leading academic medical centers.