Business Wire04.23.18
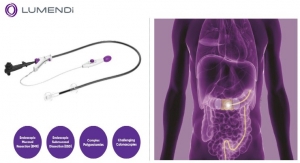
Connecticut-based medical device innovator Lumendi, LLC has announced it has received United States Food and Drug Administration (FDA) 510(k) clearance for the DiLumen C2, its second-generation endoscopic accessory indicated to ensure complete positioning of an endoscope in the large intestine and assist with optical visualization, diagnosis, and endoscopic treatment. The first product of this platform, DiLumen EIP (Endolumenal Interventional Platform), received FDA 510(k) clearance in December 2016.
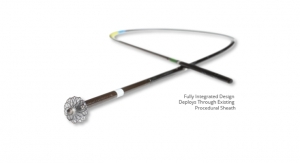
The DiLumen C2 is similar in design to the DiLumen EIP with both having two balloons used for creating a stabilizing therapeutic zone inside the colon during endolumenal interventions. The DiLumen C2 now incorporates two 6mm diameter tool channels, which accommodate two independent flexible articulating hand instruments. In 2017, Lumendi developed a flexible grasper, the DiLumen Ig that will be used in conjunction with the DiLumen C2 to grasp and manipulate mucosal tissue. Several additional flexible articulating instruments are under development and will be available for cutting and dissection of tissue. DiLumen C2, along with these flexible-articulating instruments, will create a unique therapeutic platform that will further enable clinicians to perform complex procedures completely within the colon without open surgery.
“We are continuing our commitment to develop innovative devices that facilitate endolumenal procedures for many gastrointestinal interventions in an effort to reduce costs and improve patient outcomes,” said Dr. Peter Johann, CEO of Lumendi, Ltd. “To date, we have completed over 350 procedures with the commercially available DiLumen EIP with no serious adverse events. Three clinical studies have also been completed, further demonstrating safety and cost effectiveness.” The studies are expected to be published in the near future.
DiLumen EIP facilitates endoscopic treatment of colonic lesions such as polyps, a common condition that affects millions worldwide. Such treatments may take the place of traumatic open surgical or laparoscopic procedures, potentially reducing healthcare costs. It is a single-use, close-fitting sleeve that fits securely over a standard endoscope to stabilize it in the large intestine and facilitates use of the endoscope for optical visualization, diagnosis, and treatment. The DiLumen C2 was developed by Lumendi in collaboration with the Minimally Invasive New Technologies (MINT) program at Weill Cornell Medicine and NewYork-Presbyterian Hospital. “It is the second device and the next evolution of the Endolumenal Interventional Platform based on Lumendi and MINT’s initiative to migrate many gastrointestinal surgeries to endolumenal procedures,” said Michael Parrilla, COO of Lumendi LLC.
The DiLumen C2 is similar in design to the DiLumen EIP with both having two balloons used for creating a stabilizing therapeutic zone inside the colon during endolumenal interventions. The DiLumen C2 now incorporates two 6mm diameter tool channels, which accommodate two independent flexible articulating hand instruments. In 2017, Lumendi developed a flexible grasper, the DiLumen Ig that will be used in conjunction with the DiLumen C2 to grasp and manipulate mucosal tissue. Several additional flexible articulating instruments are under development and will be available for cutting and dissection of tissue. DiLumen C2, along with these flexible-articulating instruments, will create a unique therapeutic platform that will further enable clinicians to perform complex procedures completely within the colon without open surgery.
“We are continuing our commitment to develop innovative devices that facilitate endolumenal procedures for many gastrointestinal interventions in an effort to reduce costs and improve patient outcomes,” said Dr. Peter Johann, CEO of Lumendi, Ltd. “To date, we have completed over 350 procedures with the commercially available DiLumen EIP with no serious adverse events. Three clinical studies have also been completed, further demonstrating safety and cost effectiveness.” The studies are expected to be published in the near future.
DiLumen EIP facilitates endoscopic treatment of colonic lesions such as polyps, a common condition that affects millions worldwide. Such treatments may take the place of traumatic open surgical or laparoscopic procedures, potentially reducing healthcare costs. It is a single-use, close-fitting sleeve that fits securely over a standard endoscope to stabilize it in the large intestine and facilitates use of the endoscope for optical visualization, diagnosis, and treatment. The DiLumen C2 was developed by Lumendi in collaboration with the Minimally Invasive New Technologies (MINT) program at Weill Cornell Medicine and NewYork-Presbyterian Hospital. “It is the second device and the next evolution of the Endolumenal Interventional Platform based on Lumendi and MINT’s initiative to migrate many gastrointestinal surgeries to endolumenal procedures,” said Michael Parrilla, COO of Lumendi LLC.