Business Wire12.19.17
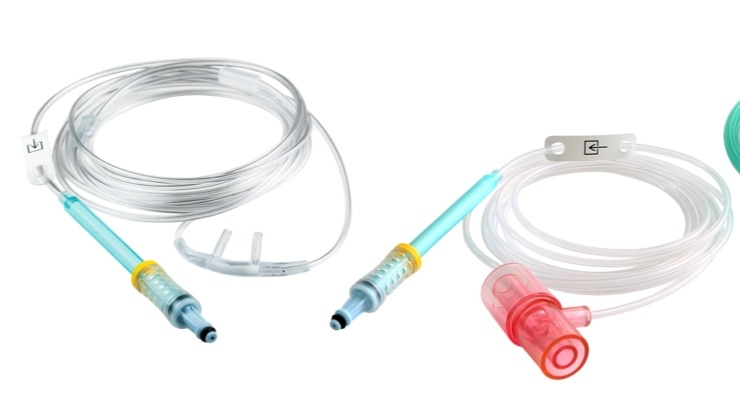
Masimo announced U.S. Food and Drug Administration(FDA) clearance and U.S. release of the full family of NomoLine capnography sampling lines. NomoLine sampling lines are available in more than 40 configurations of airway adapter sets and cannulas for use in a variety of clinical scenarios, including for intubated and non-intubated patients in both low and high humidity applications, for all patient populations, including neonatal patients. NomoLine capnography sampling lines are compatible with both Masimo and third-party OEM NomoLine monitors and enable hassle-free sidestream capnography and gas monitoring.
NomoLine “no moisture” sampling technology eliminates many common problems associated with conventional sidestream gas sampling line systems. Incorporating a unique, patented polymer, NomoLine allows water in the sampling line to continuously evaporate into the surrounding air, while leaving oxygen, carbon dioxide, and anesthetic gases unaffected. This technology eliminates the need for water traps and issues related to their handling, as well as enabling extended monitoring time in high-humidity applications, reducing the volume of disposables and the cost and waste associated with them.
NomoLine technology is designed for low-flow applications, with a very low sampling rate of 50 ml/min, supporting use on patients with low tidal volumes and high breath rates, common characteristics of neonatal patients. With functionality in any orientation, NomoLine provides a variety of sampling line options including nasal, nasal/oral, oxygen delivery, single nasal prong, and airway adapter sets. Soft, ergonomically curved cannulas help provide greater patient comfort.
NomoLine sampling lines are compatible with NomoLine ISA capnography modules, including ISA CO2, ISA AX+, and ISA OR+ for multigas monitoring. ISA modules are available on the expandable Masimo Root Patient Monitoring and Connectivity Platform through Root’s Masimo Open Connect (MOC-9) ports, as well as on more than 70 OEM monitors, including those from Spacelabs, Schiller, Ortivus, Siare, and Edan. In addition, the Rad-97 Pulse CO-Oximeter is now available with an integrated module allowing direct connection to NomoLine sampling lines.
“With NomoLine, we’ve developed innovative moisture-wicking technology —and then applied that breakthrough to an entire line of cannulas, including neonatal airway adapter sets. Masimo continues to automate noninvasive monitoring with solutions such as NomoLine that are reliable and easy to use,” said Joe Kiani, founder and CEO of Masimo.
Rad-97 and neonatal NomoLine and ISA products are currently available in the United States only.
Masimo is a global leader in noninvasive monitoring technologies. In 1995, the company debuted Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), and more recently, Oxygen Reserve Index (ORi), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7 wearable patient monitor, iSpO2 pulse oximeter for smartphones, and the MightySat fingertip pulse oximeter.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
NomoLine “no moisture” sampling technology eliminates many common problems associated with conventional sidestream gas sampling line systems. Incorporating a unique, patented polymer, NomoLine allows water in the sampling line to continuously evaporate into the surrounding air, while leaving oxygen, carbon dioxide, and anesthetic gases unaffected. This technology eliminates the need for water traps and issues related to their handling, as well as enabling extended monitoring time in high-humidity applications, reducing the volume of disposables and the cost and waste associated with them.
NomoLine technology is designed for low-flow applications, with a very low sampling rate of 50 ml/min, supporting use on patients with low tidal volumes and high breath rates, common characteristics of neonatal patients. With functionality in any orientation, NomoLine provides a variety of sampling line options including nasal, nasal/oral, oxygen delivery, single nasal prong, and airway adapter sets. Soft, ergonomically curved cannulas help provide greater patient comfort.
NomoLine sampling lines are compatible with NomoLine ISA capnography modules, including ISA CO2, ISA AX+, and ISA OR+ for multigas monitoring. ISA modules are available on the expandable Masimo Root Patient Monitoring and Connectivity Platform through Root’s Masimo Open Connect (MOC-9) ports, as well as on more than 70 OEM monitors, including those from Spacelabs, Schiller, Ortivus, Siare, and Edan. In addition, the Rad-97 Pulse CO-Oximeter is now available with an integrated module allowing direct connection to NomoLine sampling lines.
“With NomoLine, we’ve developed innovative moisture-wicking technology —and then applied that breakthrough to an entire line of cannulas, including neonatal airway adapter sets. Masimo continues to automate noninvasive monitoring with solutions such as NomoLine that are reliable and easy to use,” said Joe Kiani, founder and CEO of Masimo.
Rad-97 and neonatal NomoLine and ISA products are currently available in the United States only.
Masimo is a global leader in noninvasive monitoring technologies. In 1995, the company debuted Masimo SET Measure-through Motion and Low Perfusion pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVi), and more recently, Oxygen Reserve Index (ORi), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7 wearable patient monitor, iSpO2 pulse oximeter for smartphones, and the MightySat fingertip pulse oximeter.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.