Cohera Medical Inc. 09.18.17
Cohera Medical Inc., developer of absorbable internal surgical adhesives and sealants, has signed an exclusive, long-term distribution agreement with Terumo Corporation (Tokyo, Japan) for Sylys Surgical Sealant. Under the terms of the distribution agreement, Terumo Corporation will have the exclusive rights to market Sylys in Japan.
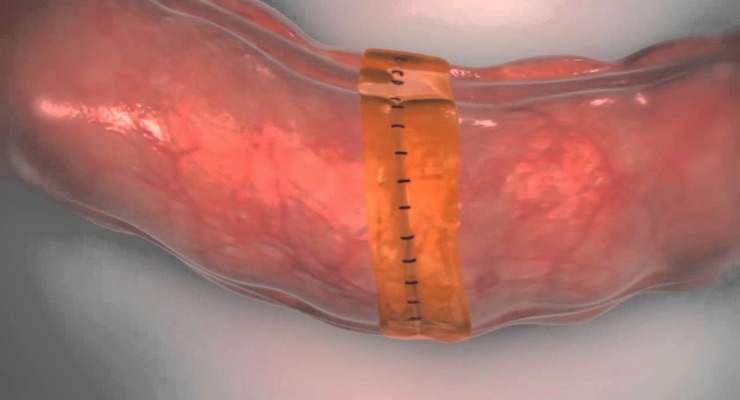
Sylys Surgical Sealant is a resorbable synthetic sealant designed to help reduce anastomotic leakage in colectomy procedures by providing additional support to the anastomosis during the first few days of healing when the development of leaks is most likely to occur. Sylys is intended to be used as an adjunct to standard closure techniques for the reinforcement and protection of anastomotic junctions.
“Entering into a distribution arrangement with such a preeminent medical device manufacturer as Terumo validates the hard work completed on Sylys Surgical Sealant, the robust market, and the enormous potential of the technology," said Patrick Daly, President and CEO of Cohera Medical, Inc. “We are looking forward to working with Terumo to bring this important lifesaving technology to the Japanese market.”
Anastomotic leakage occurs in up to 23 percent of patients undergoing colorectal surgery and is considered to be the most serious surgical complication encountered, frequently resulting in the rapid development of severe peritonitis, septic shock, multiple organ dysfunction, and death. At least one third of post-surgical deaths following colorectal surgery are attributed to leaks.
“The addition of Sylys Surgical Sealant is in perfect synergy with Terumo’s continued advancement into surgical products in Japan," said Keiko Kitamura, general manager, Surgical Division, General Hospital Company, Terumo Corporation. “We plan to work together with Cohera to move Sylys through the regulatory process in Japan as quickly as possible to the great benefit of our patients.”
The distribution agreement provides for license and marketing rights to the Sylys Surgical Sealant product for current and future indications in Japan. This represents a significant market opportunity for both companies as there are nearly a half million colorectal procedures each year collectively in the United States and Japan. To date, no other products have been approved for the reduction of colorectal leaks and Sylys is well positioned to fill this unmet medical need.
Tokyo-based Terumo Corporation is one of the world's leading medical device manufacturers with $5 billion in sales and operations in more than 160 nations. Founded in 1921, the company develops, manufactures, and distributes medical devices, including products for use in cardiothoracic surgery, interventional procedures, and transfusion medicine. The company also manufactures an array of syringe and hypodermic needle products for hospital and physician office use.
Cohera Medical Inc. is developing and commercializing a line of surgical adhesives and sealants. Cohera Medical’s products are based on a unique chemical design that is resorbable, non-toxic, and easy to use. The company’s lead product, TissuGlu Surgical Adhesive, is indicated for use in the United States for the approximation of tissue planes in abdominoplasty procedures. TissuGlu is currently approved for sale in the European Union for the approximation of tissue layers where subcutaneous dead space exists between the tissue planes in large flap surgical procedures such as abdominoplasty and is being utilized in Europe to eliminate drains or reduce complications in patients undergoing large flap surgical procedures such as abdominoplasty, mastectomy, ventral hernia repair, decubitus and latissimus dorsi flap procedures. The company’s second product under development, Sylys Surgical Sealant, the first synthetic sealant designed specifically to help reduce anastomotic leaks, has received CE Mark approval in Europe as an adjunct to standard closure in ileostomy reversal procedures. TissuGlu and Sylys are the first products in a pipeline of technology that includes adhesives for surgical mesh fixation, meniscal repair, and other orthopedic indications. Sylys and the other Cohera Medical products are currently available for investigational use only and have not yet been approved for sale by the U.S. Food and Drug Administration.
Sylys Surgical Sealant is a resorbable synthetic sealant designed to help reduce anastomotic leakage in colectomy procedures by providing additional support to the anastomosis during the first few days of healing when the development of leaks is most likely to occur. Sylys is intended to be used as an adjunct to standard closure techniques for the reinforcement and protection of anastomotic junctions.
“Entering into a distribution arrangement with such a preeminent medical device manufacturer as Terumo validates the hard work completed on Sylys Surgical Sealant, the robust market, and the enormous potential of the technology," said Patrick Daly, President and CEO of Cohera Medical, Inc. “We are looking forward to working with Terumo to bring this important lifesaving technology to the Japanese market.”
Anastomotic leakage occurs in up to 23 percent of patients undergoing colorectal surgery and is considered to be the most serious surgical complication encountered, frequently resulting in the rapid development of severe peritonitis, septic shock, multiple organ dysfunction, and death. At least one third of post-surgical deaths following colorectal surgery are attributed to leaks.
“The addition of Sylys Surgical Sealant is in perfect synergy with Terumo’s continued advancement into surgical products in Japan," said Keiko Kitamura, general manager, Surgical Division, General Hospital Company, Terumo Corporation. “We plan to work together with Cohera to move Sylys through the regulatory process in Japan as quickly as possible to the great benefit of our patients.”
The distribution agreement provides for license and marketing rights to the Sylys Surgical Sealant product for current and future indications in Japan. This represents a significant market opportunity for both companies as there are nearly a half million colorectal procedures each year collectively in the United States and Japan. To date, no other products have been approved for the reduction of colorectal leaks and Sylys is well positioned to fill this unmet medical need.
Tokyo-based Terumo Corporation is one of the world's leading medical device manufacturers with $5 billion in sales and operations in more than 160 nations. Founded in 1921, the company develops, manufactures, and distributes medical devices, including products for use in cardiothoracic surgery, interventional procedures, and transfusion medicine. The company also manufactures an array of syringe and hypodermic needle products for hospital and physician office use.
Cohera Medical Inc. is developing and commercializing a line of surgical adhesives and sealants. Cohera Medical’s products are based on a unique chemical design that is resorbable, non-toxic, and easy to use. The company’s lead product, TissuGlu Surgical Adhesive, is indicated for use in the United States for the approximation of tissue planes in abdominoplasty procedures. TissuGlu is currently approved for sale in the European Union for the approximation of tissue layers where subcutaneous dead space exists between the tissue planes in large flap surgical procedures such as abdominoplasty and is being utilized in Europe to eliminate drains or reduce complications in patients undergoing large flap surgical procedures such as abdominoplasty, mastectomy, ventral hernia repair, decubitus and latissimus dorsi flap procedures. The company’s second product under development, Sylys Surgical Sealant, the first synthetic sealant designed specifically to help reduce anastomotic leaks, has received CE Mark approval in Europe as an adjunct to standard closure in ileostomy reversal procedures. TissuGlu and Sylys are the first products in a pipeline of technology that includes adhesives for surgical mesh fixation, meniscal repair, and other orthopedic indications. Sylys and the other Cohera Medical products are currently available for investigational use only and have not yet been approved for sale by the U.S. Food and Drug Administration.