PR Newswire05.25.17
Boston Scientific announced positive results from the Post Approval Clinical Trial Evaluating Bronchial Thermoplasty (BT) in Severe Persistent Asthma (PAS2) study evaluating patients treated with the Alair System. The data, presented at the American Thoracic Society International Conference in Washington, D.C., demonstrate that BT reduces complications in adult patients with severe persistent asthma.
PAS2 is an open-label study that enrolled 284 patients at 27 research centers in the United States and Canada. Study participants have asthma that is not well controlled by inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA) and may also rely heavily on additional maintenance medications including oral corticosteroids (OCS) and biologics. At the beginning of the study, PAS2 study participants were, on average, 45.7 years old with a body mass index of 32.2 kg/m2, took mean ICS and LABA doses of 2275 µg/day and 106 µg/day, respectively, with 19.4 percent utilizing OCS and 15.8 percent omalizumab. These measures are significantly higher than in prior studies.
Overall, patients in the study showed marked clinical improvement that was sustained for two years following treatment. The data demonstrated that:
PAS2 study participants will be followed for five years post treatment to conduct further analysis.
"The findings of the PAS2 study provide important real-world evidence that patients with poorly controlled severe asthma on high doses of medications, including biologics, experience significant and sustained improvements in asthma control following BT," said Geoffrey Chupp, M.D., principal investigator and director, Yale Center for Asthma and Airways Disease, Yale University School of Medicine, New Haven, Connecticut. "These results reinforce previously published data from randomized controlled studies and demonstrate that BT delivered by the Alair System is both safe and effective for a wide range of patients with severe asthma."
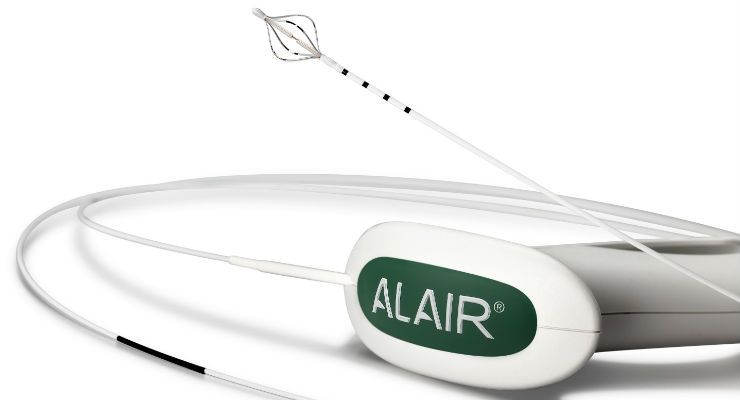
The Alair System for BT was approved by the U.S. Food and Drug Administration (FDA) in 2010 and is the first non-pharmacologic, device-based treatment for severe, persistent asthma. The Alair System delivers controlled thermal energy to the airway wall to reduce the amount of excess smooth muscle tissue in the airways. With less smooth muscle, the airways constrict less, reducing severe asthma attacks and making breathing easier.
"Bronchial Thermoplasty is an established treatment that can transform the lives of people with severe asthma," said Art Butcher, senior vice president and president, Endoscopy, Boston Scientific. "We are committed to working with the healthcare community to ensure that patients with severe asthma have access to this important treatment option."
Asthma is a chronic inflammatory disease of the airways characterized by recurrent attacks of breathlessness and wheezing, which vary in severity and frequency from person to person. Asthma currently affects more than 25 million people and approximately 10 percent of cases are considered severe.1,2 For those who suffer from severe asthma, even the highest dose of standard medications may not alleviate the risk of frequent and life-threatening asthma attacks. During an asthma attack, the lining of the bronchial tubes swell, causing the airways to narrow and reducing the flow of air in and out of the lungs.3
References
1"Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 01 May 2012. Web. 05 May 2017. <https://www.cdc.gov/nchs/products/databriefs/db94.htm>.
2"The Prevalence of Severe Refractory Asthma | AAAAI." The American Academy of Allergy, Asthma & Immunology. The Journal of Allergy and Clinical Immunology, Oct. 2014. Web. 05 May 2017. <https://www.aaaai.org/global/latest-research-summaries/New-Research-from-JACI-In-Practice/refractory-asthma>.
3WHO. Asthma Fact sheet N°307. November 2013. Available at: http://www.who.int/mediacentre/factsheets/fs307/en/index.html (accessed December 2016).
PAS2 is an open-label study that enrolled 284 patients at 27 research centers in the United States and Canada. Study participants have asthma that is not well controlled by inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA) and may also rely heavily on additional maintenance medications including oral corticosteroids (OCS) and biologics. At the beginning of the study, PAS2 study participants were, on average, 45.7 years old with a body mass index of 32.2 kg/m2, took mean ICS and LABA doses of 2275 µg/day and 106 µg/day, respectively, with 19.4 percent utilizing OCS and 15.8 percent omalizumab. These measures are significantly higher than in prior studies.
Overall, patients in the study showed marked clinical improvement that was sustained for two years following treatment. The data demonstrated that:
- The percentage of study participants that had at least one severe asthma exacerbation decreased from 77.8 percent in the year prior to treatment to 50.4 percent in year one and 46.4 percent in year two;
- The percentage of patients who had asthma-related hospitalizations decreased from 16.1 percent in the year prior to BT to 8 percent and 7.3 percent in years one and two following treatment;
- The percentage of patients with asthma-related ER visits reduced from 29.4 percent in the year before BT to 18.3 percent and 14.5 percent in years one and two post-BT;
- By the second year following BT treatment, the percentage of patients taking OCS to manage their asthma symptoms had decreased by 39.2 percent from 19.4 percent pre-treatment to 11.8 percent.
PAS2 study participants will be followed for five years post treatment to conduct further analysis.
"The findings of the PAS2 study provide important real-world evidence that patients with poorly controlled severe asthma on high doses of medications, including biologics, experience significant and sustained improvements in asthma control following BT," said Geoffrey Chupp, M.D., principal investigator and director, Yale Center for Asthma and Airways Disease, Yale University School of Medicine, New Haven, Connecticut. "These results reinforce previously published data from randomized controlled studies and demonstrate that BT delivered by the Alair System is both safe and effective for a wide range of patients with severe asthma."
The Alair System for BT was approved by the U.S. Food and Drug Administration (FDA) in 2010 and is the first non-pharmacologic, device-based treatment for severe, persistent asthma. The Alair System delivers controlled thermal energy to the airway wall to reduce the amount of excess smooth muscle tissue in the airways. With less smooth muscle, the airways constrict less, reducing severe asthma attacks and making breathing easier.
"Bronchial Thermoplasty is an established treatment that can transform the lives of people with severe asthma," said Art Butcher, senior vice president and president, Endoscopy, Boston Scientific. "We are committed to working with the healthcare community to ensure that patients with severe asthma have access to this important treatment option."
Asthma is a chronic inflammatory disease of the airways characterized by recurrent attacks of breathlessness and wheezing, which vary in severity and frequency from person to person. Asthma currently affects more than 25 million people and approximately 10 percent of cases are considered severe.1,2 For those who suffer from severe asthma, even the highest dose of standard medications may not alleviate the risk of frequent and life-threatening asthma attacks. During an asthma attack, the lining of the bronchial tubes swell, causing the airways to narrow and reducing the flow of air in and out of the lungs.3
References
1"Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 01 May 2012. Web. 05 May 2017. <https://www.cdc.gov/nchs/products/databriefs/db94.htm>.
2"The Prevalence of Severe Refractory Asthma | AAAAI." The American Academy of Allergy, Asthma & Immunology. The Journal of Allergy and Clinical Immunology, Oct. 2014. Web. 05 May 2017. <https://www.aaaai.org/global/latest-research-summaries/New-Research-from-JACI-In-Practice/refractory-asthma>.
3WHO. Asthma Fact sheet N°307. November 2013. Available at: http://www.who.int/mediacentre/factsheets/fs307/en/index.html (accessed December 2016).