Wyss Institute02.01.17
Enteroviruses enter the human body through the digestive or respiratory tract and from there spread to other sites in the body where they can cause a variety of serious health threats including meningitis, pancreatitis, myocarditis, the death of motor neurons, and perhaps even help trigger diabetes. However, they remain a challenge to study because they cannot be grown in conventional human cell cultures. Yet, understanding how enteroviruses invade gastrointestinal cells, multiply within them, and are released to other sites in the body could be key to ending the present dearth of specific anti-viral therapies and vaccines.
Towards solving this problem, a multidisciplinary team of tissue engineers and biologists at Harvard’s Wyss Institute for Biologically Inspired Engineering working alongside scientists from the Molecular Virology Team at the U.S. Food and Drug Administration (FDA)’s Center for Food Safety and Applied Nutrition now have leveraged the Wyss Institute’s previously developed human gut-on-a-chip to mimic the entry, host cell-interaction and multiplication of a pathogenic clinical strain of Coxsackievirus using gut epithelium outside the human body. Their findings are reported in PLoS One.
“We teamed up with FDA researchers to show for the first time that an enterovirus can be successfully cultured in a microfluidic human Gut Chip system. We were excited to find that the organ-on-a-chip approach offers a potential new way to study these viral pathogens under more physiologically relevant conditions in vitro,” said the Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who led the team. Ingber also is the Judah Folkman Professor of Vascular Biology at Boston Children’s Hospital and Harvard Medical School, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS).
“Now that we have a functional minimal system in place that replicates typical host-pathogen interactions, we can start to vary the type of intestinal cells, include immune cells that may contribute to the host response to infection, or create tissues using human stem cell-derived intestinal cells to tease out virus specificities and requirements for infection,” said Ingber.
First developed in 2012, the Wyss Institute’s human gut-on-a-chip is a transparent, hollow-channeled microfluidic device the size of a computer memory stick that recapitulates the gut microenvironment. Human intestinal epithelial cells are cultured in a microchannel on a porous membrane that separates them from a parallel microchannel that mimics a neighboring capillary blood vessel. Fluid with or without viruses is flowed through both channels and exchanged through the pores of the membrane. Suction forces are also applied to parallel hollow channels, which produce cyclic deformations in the tissue that mimic intestinal peristalsis-like motions. This culture approach results in the growth of a fully differentiated gut epithelium that exhibits three-dimensional finger-like villus structures and that harbors all of the relevant cell types of the small intestine. In 2015, the team added more complexity to their biomimicking device by co-culturing a capillary endothelium on the lower surface of the membrane as well as a bacterial gut microbiome on the lumen of the epithelial channel to model aspects of human intestinal inflammation.
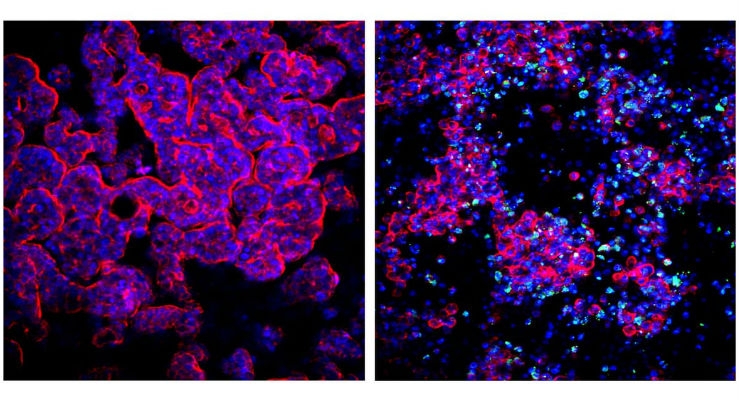
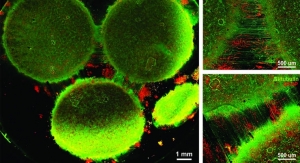
“We were able to recapitulate how Coxsackievirus B1 enters the epithelium lining the intestinal villi from the gut lumen, and show that the virus replicates inside the cells and exits them again via a specific route to go on to infect cells downstream in the channel,” said Remi Villenave, Ph.D., the study’s first author who did the work when he was a postdoctoral fellow working with Ingber. “Also inflammatory cytokines that likely contribute to intestinal tissue injury in the chip were preferentially secreted into the lumen of the intestinal channel rather than into the media transporting channel, paralleling what is seen in acute infections in people.”
Besides Villenave and Ingber, the article is also authored by FDA researchers Samantha Wales, Efstathia Papafragkou, Christopher Elkins and Michael Kulka. Additional authors are Tiama Hamkins-Indik, James Weaver, Thomas Ferrante and Anthony Bahinski, who at the time of the study were affiliated with the Wyss Institute.
Towards solving this problem, a multidisciplinary team of tissue engineers and biologists at Harvard’s Wyss Institute for Biologically Inspired Engineering working alongside scientists from the Molecular Virology Team at the U.S. Food and Drug Administration (FDA)’s Center for Food Safety and Applied Nutrition now have leveraged the Wyss Institute’s previously developed human gut-on-a-chip to mimic the entry, host cell-interaction and multiplication of a pathogenic clinical strain of Coxsackievirus using gut epithelium outside the human body. Their findings are reported in PLoS One.
“We teamed up with FDA researchers to show for the first time that an enterovirus can be successfully cultured in a microfluidic human Gut Chip system. We were excited to find that the organ-on-a-chip approach offers a potential new way to study these viral pathogens under more physiologically relevant conditions in vitro,” said the Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who led the team. Ingber also is the Judah Folkman Professor of Vascular Biology at Boston Children’s Hospital and Harvard Medical School, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS).
“Now that we have a functional minimal system in place that replicates typical host-pathogen interactions, we can start to vary the type of intestinal cells, include immune cells that may contribute to the host response to infection, or create tissues using human stem cell-derived intestinal cells to tease out virus specificities and requirements for infection,” said Ingber.
First developed in 2012, the Wyss Institute’s human gut-on-a-chip is a transparent, hollow-channeled microfluidic device the size of a computer memory stick that recapitulates the gut microenvironment. Human intestinal epithelial cells are cultured in a microchannel on a porous membrane that separates them from a parallel microchannel that mimics a neighboring capillary blood vessel. Fluid with or without viruses is flowed through both channels and exchanged through the pores of the membrane. Suction forces are also applied to parallel hollow channels, which produce cyclic deformations in the tissue that mimic intestinal peristalsis-like motions. This culture approach results in the growth of a fully differentiated gut epithelium that exhibits three-dimensional finger-like villus structures and that harbors all of the relevant cell types of the small intestine. In 2015, the team added more complexity to their biomimicking device by co-culturing a capillary endothelium on the lower surface of the membrane as well as a bacterial gut microbiome on the lumen of the epithelial channel to model aspects of human intestinal inflammation.
“We were able to recapitulate how Coxsackievirus B1 enters the epithelium lining the intestinal villi from the gut lumen, and show that the virus replicates inside the cells and exits them again via a specific route to go on to infect cells downstream in the channel,” said Remi Villenave, Ph.D., the study’s first author who did the work when he was a postdoctoral fellow working with Ingber. “Also inflammatory cytokines that likely contribute to intestinal tissue injury in the chip were preferentially secreted into the lumen of the intestinal channel rather than into the media transporting channel, paralleling what is seen in acute infections in people.”
Besides Villenave and Ingber, the article is also authored by FDA researchers Samantha Wales, Efstathia Papafragkou, Christopher Elkins and Michael Kulka. Additional authors are Tiama Hamkins-Indik, James Weaver, Thomas Ferrante and Anthony Bahinski, who at the time of the study were affiliated with the Wyss Institute.