Vascular Dynamics02.01.17
Vascular Dynamics Inc. (VDI), a privately held medical device company developing novel solutions for the treatment of hypertension, has announced that the United States Food and Drug Administration (FDA) has approved the company’s application to participate in the Expedited Access Pathway (EAP) program for its MobiusHD device for the treatment of resistant hypertension.
VDI was also one of only nine companies chosen for the FDA’s Early Feasibility Study Investigational Device Exemptions (IDE) Pilot Program, in 2012. The Pilot Program enables companies to conduct smaller-scale studies under the guidance of the Agency in the United States in order to meet the requirements for an earlier pathway toward approval.
The EAP program is a focused initiative, recently launched by FDA, to significantly accelerate access for US patients and their physicians to innovative medical treatments. The program was designed for certain medical devices that demonstrate the potential to address unmet medical needs for life-threatening or irreversibly debilitating diseases or conditions, such as resistant hypertension, that may be subject to premarket approval applications.
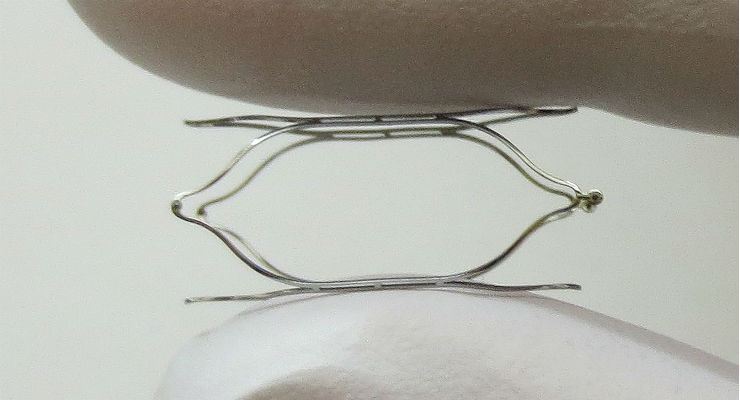
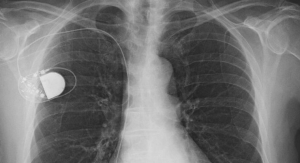
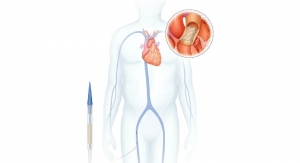
The MobiusHD System, a minimally-invasive system for the treatment of resistant hypertension, capitalizes on the ability of the body’s baroreceptor mechanism to regulate blood pressure. Baroreceptors are receptors located in the carotid artery that sense blood pressure and relay that information to the brain. The MobiusHD implant is designed to amplify the signals received by the surrounding arterial baroreceptors, and thereby amplify the body’s natural response to lower blood pressure through vasodilation.
“Among the benefits of the technology are that it may reduce ambulatory blood pressure in resistant hypertension patients who have not benefited from other device or drug based treatments,” said Professor Brian Williams, Co-Principal Investigator of the CALM II worldwide clinical trial, and NIHR University College London Hospitals Biomedical Research Centre Director. “The Mobius HD technology may provide an important solution for those patients. We are enthusiastic about the product’s potential as we have seen tremendous results in early studies in the US and Europe.”
“The EAP designation is an incredible opportunity to accelerate the path to approval in the United States, and we are honored to have been chosen to be among the small handful of devices that have met the FDA’s requirements,” said Robert Stern, president and CEO of Vascular Dynamics. “We now look forward to working closely with the FDA to try to reduce the time to get innovative devices to the patients that need them, while still maintaining the high standards of safety, efficacy and scientific validity required by the program.”
“In clinical practice, we regularly observe that despite multiple treatment modalities, many patients are still not responding adequately to therapy, whether it be lifestyle changes, multiple drug therapy, or the combination of each. This lack of response significantly increases their risk of further complications,” said Gregg Stone, MD, co-principal investigator of the CALM II worldwide clinical trial, director of cardiovascular research and education for Columbia University Medical Center/New York-Presbyterian Hospital, and co-director of medical research and education at the Cardiovascular Research Foundation.
“The EAP Designation for the MobiusHD device comes at a critical time for hypertension physicians, who are actively looking for treatment options for patients who are not adequately managed with current guideline-directed therapies,” said George Bakris, MD, chairman of the worldwide CALM II clinical trial hypertension committee, and director, ASH Comprehensive Hypertension Center, The University of Chicago School of Medicine.
VDI was also one of only nine companies chosen for the FDA’s Early Feasibility Study Investigational Device Exemptions (IDE) Pilot Program, in 2012. The Pilot Program enables companies to conduct smaller-scale studies under the guidance of the Agency in the United States in order to meet the requirements for an earlier pathway toward approval.
The EAP program is a focused initiative, recently launched by FDA, to significantly accelerate access for US patients and their physicians to innovative medical treatments. The program was designed for certain medical devices that demonstrate the potential to address unmet medical needs for life-threatening or irreversibly debilitating diseases or conditions, such as resistant hypertension, that may be subject to premarket approval applications.
The MobiusHD System, a minimally-invasive system for the treatment of resistant hypertension, capitalizes on the ability of the body’s baroreceptor mechanism to regulate blood pressure. Baroreceptors are receptors located in the carotid artery that sense blood pressure and relay that information to the brain. The MobiusHD implant is designed to amplify the signals received by the surrounding arterial baroreceptors, and thereby amplify the body’s natural response to lower blood pressure through vasodilation.
“Among the benefits of the technology are that it may reduce ambulatory blood pressure in resistant hypertension patients who have not benefited from other device or drug based treatments,” said Professor Brian Williams, Co-Principal Investigator of the CALM II worldwide clinical trial, and NIHR University College London Hospitals Biomedical Research Centre Director. “The Mobius HD technology may provide an important solution for those patients. We are enthusiastic about the product’s potential as we have seen tremendous results in early studies in the US and Europe.”
“The EAP designation is an incredible opportunity to accelerate the path to approval in the United States, and we are honored to have been chosen to be among the small handful of devices that have met the FDA’s requirements,” said Robert Stern, president and CEO of Vascular Dynamics. “We now look forward to working closely with the FDA to try to reduce the time to get innovative devices to the patients that need them, while still maintaining the high standards of safety, efficacy and scientific validity required by the program.”
“In clinical practice, we regularly observe that despite multiple treatment modalities, many patients are still not responding adequately to therapy, whether it be lifestyle changes, multiple drug therapy, or the combination of each. This lack of response significantly increases their risk of further complications,” said Gregg Stone, MD, co-principal investigator of the CALM II worldwide clinical trial, director of cardiovascular research and education for Columbia University Medical Center/New York-Presbyterian Hospital, and co-director of medical research and education at the Cardiovascular Research Foundation.
“The EAP Designation for the MobiusHD device comes at a critical time for hypertension physicians, who are actively looking for treatment options for patients who are not adequately managed with current guideline-directed therapies,” said George Bakris, MD, chairman of the worldwide CALM II clinical trial hypertension committee, and director, ASH Comprehensive Hypertension Center, The University of Chicago School of Medicine.