Stefano Longo, Product Manager, VSi Parylene07.19.22
As demonstrated in the recent MPO article, Discovering the Value of Surface Treatments and Coatings for Medtech, parylene coatings are often an overlooked yet powerful surface treatment solution for medical technologies—especially as medical devices become smaller and more compact.
Parylene has long been beneficial in medical applications—protecting and enhancing a wide array of devices and components, including cardiac assist devices, electrosurgical tools, cochlear and ocular implants, neurostimulation devices, stents, catheters, elastomeric seals, mandrels, and more.
Various medical coating options are available, each with its own set of properties and characteristics. Parylene, however, offers unique material properties and molecular-level vapor deposition that can be especially advantageous for many surface applications and delicate devices.
One of the first considerations in selecting a surface treatment for medical device manufacturers is whether or not a material is biocompatible and biostable. Any surface that comes into direct and extended contact with body tissue, body fluids, proteins, enzymes, or lipids must be biostable. If it is not, the surface must be safeguarded in a manner that does not negatively affect its therapeutic value.
Parylene coatings are both biocompatible and biostable and have been tested according to both the Biological Evaluation requirements of ISO 10993 and U.S. Pharmacopeia Class VI. Additionally, parylene has demonstrated safe and effective use in a wide range of medical coating applications over the past five decades.
Parylene films possess very low blood clotting properties and low potential for triggering an immune response. In addition, the film also forms an effective barrier against the passage of contaminants from a coated substrate to the body or the body to a coated substrate. The types of biocompatibility-related testing that parylene has passed are shown in Table 1.
Sterilization Resistance
Sterilization is intended to completely destroy all microbial life-forms, including viruses, bacteria, and fungi on the surface of a medical device. This process can be accomplished by a number of chemical or physical means. The difficulty in sterilization is in attaining the balance between rendering a surface sterile without destroying or degrading the useful life of either the sterilized part or its coating. Parylene coatings offer excellent moisture, chemical, dielectric, thermal, and UV stability, which allows it to withstand multiple sterilization methods including steam, E-beam, gamma ray, EtO, and autoclave. These various sterilization methods and their effects on the material properties of parylene N and C are shown in Table 2.
Lubricity
Lubricity is the measure of the reduction in the coefficient of friction (CoF) and/or wear by a biocompatible lubricant. In biomedical materials and implants, the wear performance may be related to their apparent CoF in the presence of biological fluids.
Parylene acts as a low-friction polymer coating that allows for easy sliding acting as a dry lubricant. Lubricity is important in many medical applications because increased friction typically means a procedure is more harmful to tissue and takes longer to accomplish. Parylene is about as slippery as Teflon and has proven to be extremely useful for stents, syringes, catheters, needles, and other medical implants (Table 3).
A three-stage, vacuum, and thermal vapor-deposition process converts the raw material, called di-paraxylylene or dimer, from a white powder into a transparent polymer film. During this process, parylene deposits molecule by molecule onto parts placed inside a vacuum chamber. This produces an extremely conformal coating that evenly covers grooves, crevices, gaps, and even sharp points. Because the coating is applied molecule by molecule, the thickness is highly controllable down to the micron level. Since parylene is never a liquid at any stage in its deposition process, it does not exhibit the viscous effects of pooling, bridging, or peeling found with liquid coatings (Figure 1).
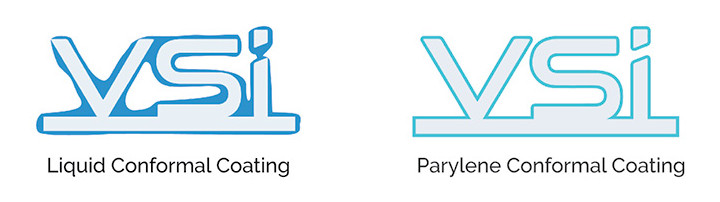
Figure 1: Since parylene is never a liquid at any stage in its deposition process, it does not exhibit the viscous effects of pooling, bridging, or peeling found with liquid coatings.
Nevertheless, as reliance on any component increases, including surface treatments, so too does the potential for supply disruptions to have a major impact on production. This is because in highly regulated industries, like medical devices, components and their suppliers must go through an involved approval process to ensure regulatory compliance. If a component is in short supply, MDMs cannot simply replace the part or its supplier with a similar component to avoid supply disruptions. Parylene is no exception.
Like most engineered surface treatments, parylene has been offered almost exclusively as an outsourced service, requiring parts to be shipped back and forth between an MDM and supplier. As the reverberating impacts of the COVID-19 pandemic continue to be felt, MDMs are increasingly pressed to reduce the level of supply risk in their supply chains. This, in turn, has led to a demand for greater access to critical surface treatments, either through more flexible secondary sources or by transferring surface treatment production onto their own factory floor.
VSi Parylene is simplifying this risk mitigation process by offering forward-thinking products and services that meet the specific needs of the MDM supply environment. For MDMs who require that parts never leave their hands or who want maximum control, VSi provides a stand-alone parylene production system that is easily transferable. This includes optional installation, staff training, as well as process setup and support. This can help customers establish a qualified process that produces high-quality film coatings according to their unique specifications. For MDMs who need a phased transition, flexible hybrid coating services allow customers to begin by outsourcing parylene coating at first and later bring the process in-house.
Stefano Longo is a product manager at VSi Parylene, a provider of parylene coatings and innovative technology solutions. He believes parylene has been underutilized in multiple industries and could be the key to unlocking the next milestone in your product’s lifecycle. Functioning as the junction between product management, applications engineering, and customer service, Longo helps consult customers on how parylene can benefit their device and provide longer lifespans to mission-critical devices. He holds a BA in environmental science and geology, and is currently working on a Master of Business Administration, both from the University of Colorado. Previous work experience include positions within the renewable and finance sectors.
Parylene has long been beneficial in medical applications—protecting and enhancing a wide array of devices and components, including cardiac assist devices, electrosurgical tools, cochlear and ocular implants, neurostimulation devices, stents, catheters, elastomeric seals, mandrels, and more.
Various medical coating options are available, each with its own set of properties and characteristics. Parylene, however, offers unique material properties and molecular-level vapor deposition that can be especially advantageous for many surface applications and delicate devices.
Advanced Material Properties
Biocompatibility and BiostabilityOne of the first considerations in selecting a surface treatment for medical device manufacturers is whether or not a material is biocompatible and biostable. Any surface that comes into direct and extended contact with body tissue, body fluids, proteins, enzymes, or lipids must be biostable. If it is not, the surface must be safeguarded in a manner that does not negatively affect its therapeutic value.
Parylene coatings are both biocompatible and biostable and have been tested according to both the Biological Evaluation requirements of ISO 10993 and U.S. Pharmacopeia Class VI. Additionally, parylene has demonstrated safe and effective use in a wide range of medical coating applications over the past five decades.
Parylene films possess very low blood clotting properties and low potential for triggering an immune response. In addition, the film also forms an effective barrier against the passage of contaminants from a coated substrate to the body or the body to a coated substrate. The types of biocompatibility-related testing that parylene has passed are shown in Table 1.
| Study | Standard | Parylene Type | Result |
| ASTM Hemolysis Complete (Direct and Indirect) | ISO 10993-4 | C & N | Meets Requirements |
| ISO Partial Thromboplastin Time | ISO 10993-4 | C & N | Meets Requirements |
| ISO Lee & White Clotting Time – Human Blood (Direct) | ISO 10993-4 | C & N | Meets Requirements |
| ISO Lee & White Clotting Time – Human Blood (Indirect) | ISO 10993-4 | C & N | Meets Requirements |
| ISO In Vitro Hemocompatibility (Direct) | ISO 10993-4 | C & N | Meets Requirements |
| ISO In Vitro Hemocompatibility (Indirect) | ISO 10993-4 | C & N | Meets Requirements |
| ISO Cytotoxicity Test – Neutral Red Uptake 4 Concentrations | ISO 10993-5 | C & N | Meets Requirements |
| ISO MEM Elution Cytotoxicity | ISO 10993-5 | C & N | Extracts Confirm Suitability |
| ISO Implant/Muscle/2Weeks | ISO 10993-6 | C & N | Classified as Non-Irritant |
| ISO Implant/Muscle/13Weeks | ISO 10993-6 | C & N | Classified as Non-Irritant |
| ISO Implant/Muscle/26Weeks | ISO 10993-6 | C & N | Classified as Non-Irritant |
| ISO Klingman Maximization/2 Extracts/35 Animals/Concurrent (+) controls | ISO 10993-10 | C & N | Meets Requirements |
| ISO Rabbit Pyrogen-Material Mediated | ISO 10993-11 | C & N | Meets Requirements |
| USP Physiochemical/Plastics | USP | C & N | Meets Criteria |
| USP Physiochemical Test For Plastics – Non-Volatile Residue | USP | C & N | Meets Criteria |
| USP Class VI Test Parylene C | USP | C | Meets Criteria |
| USP Class VI Test Parylene N | USP | N | Meets Criteria |
| RoHS Compliance Parylene Type C | EU | C | Compliant |
| RoHS Compliance Parylene Type N | EU | N | Compliant |
| Reach Compliance Testing Per Regulation 1907/2006 Parylene C | ECHA | C | Passes |
| Reach Compliance Testing Per Regulation 1907/2006 Parylene N | ECHA | N | Passes |
Table 1: Parylene coating and dimer testing based on industry literature.
Sterilization Resistance
Sterilization is intended to completely destroy all microbial life-forms, including viruses, bacteria, and fungi on the surface of a medical device. This process can be accomplished by a number of chemical or physical means. The difficulty in sterilization is in attaining the balance between rendering a surface sterile without destroying or degrading the useful life of either the sterilized part or its coating. Parylene coatings offer excellent moisture, chemical, dielectric, thermal, and UV stability, which allows it to withstand multiple sterilization methods including steam, E-beam, gamma ray, EtO, and autoclave. These various sterilization methods and their effects on the material properties of parylene N and C are shown in Table 2.
|
Sterilization Method |
Parylene N | Parylene C | ||||||||
| Dielectric Strength | WVT | Tensile Strength | Tensile Modulus | COF | Dielectric Strength | WVT | Tensile Strength | Tensile Modulus | COF | |
| Steam | None | Δ43% | None | Δ12% | Δ38% | None | Δ5* | Δ17% | Δ9% | None |
| EtO | None | Δ21% | None | None | Δ33% | None | 8% | None | None | None |
| E-beam | NA | None | None | None | None | NA | None | None | None | None |
| H2O2 plasma | None | None | None | None | Δ48% | Δ9% | None | None | None | Δ188% |
| Gamma | None | None | None | None | None | None | Δ5% | None | None | None |
Table 2: Effects of various sterilization methods on parylene material properties.
*5% values aren’t likely to be statistically significant.
NA = not applicable
COF = coefficient of friction
WVT = water vapor transmission
*5% values aren’t likely to be statistically significant.
NA = not applicable
COF = coefficient of friction
WVT = water vapor transmission
Lubricity
Lubricity is the measure of the reduction in the coefficient of friction (CoF) and/or wear by a biocompatible lubricant. In biomedical materials and implants, the wear performance may be related to their apparent CoF in the presence of biological fluids.
Parylene acts as a low-friction polymer coating that allows for easy sliding acting as a dry lubricant. Lubricity is important in many medical applications because increased friction typically means a procedure is more harmful to tissue and takes longer to accomplish. Parylene is about as slippery as Teflon and has proven to be extremely useful for stents, syringes, catheters, needles, and other medical implants (Table 3).
| Mechanical Properties | Parylene C | Parylene N | Parylene F |
|
Coefficient of Friction The coefficient of friction is the minimum force required to get an object to slide on a surface, divided by the forces pressing them together. It is important to note that the difference in static and dynamic coefficient of friction is undetectable for parylene. |
0.29 static and dynamic | 0.25 static and dynamic |
0.35 static 0.39 dynamic |
Table 3: Coefficient of friction value for Parylene C, N, F.
Parylene Deposition Process
The way parylene is applied to devices makes parylene films particularly attractive for medical applications. Parylene’s unique vapor polymerization deposition produces near-perfect conformance, pinhole-free coverage at very thin layers, and the ability to penetrate and coat complex surfaces. In contrast to conventional coatings, parylene deposits onto objects at room temperature as a vapor and not a liquid. As a result, it does not require the use of any solvents, catalysts, plasticizers, or any other additives to achieve total encapsulation.A three-stage, vacuum, and thermal vapor-deposition process converts the raw material, called di-paraxylylene or dimer, from a white powder into a transparent polymer film. During this process, parylene deposits molecule by molecule onto parts placed inside a vacuum chamber. This produces an extremely conformal coating that evenly covers grooves, crevices, gaps, and even sharp points. Because the coating is applied molecule by molecule, the thickness is highly controllable down to the micron level. Since parylene is never a liquid at any stage in its deposition process, it does not exhibit the viscous effects of pooling, bridging, or peeling found with liquid coatings (Figure 1).

Figure 1: Since parylene is never a liquid at any stage in its deposition process, it does not exhibit the viscous effects of pooling, bridging, or peeling found with liquid coatings.
Parylene Surface Treatments Supply for Medtech
As the demand for devices that are smaller, more complex, and have greater functionality grows, medical device manufacturers (MDMs) look to engineered surface treatments to help ensure safe operation of new technologies. In fact, surface treatments are increasingly among the top critical components required for enabling new medical devices.Nevertheless, as reliance on any component increases, including surface treatments, so too does the potential for supply disruptions to have a major impact on production. This is because in highly regulated industries, like medical devices, components and their suppliers must go through an involved approval process to ensure regulatory compliance. If a component is in short supply, MDMs cannot simply replace the part or its supplier with a similar component to avoid supply disruptions. Parylene is no exception.
Like most engineered surface treatments, parylene has been offered almost exclusively as an outsourced service, requiring parts to be shipped back and forth between an MDM and supplier. As the reverberating impacts of the COVID-19 pandemic continue to be felt, MDMs are increasingly pressed to reduce the level of supply risk in their supply chains. This, in turn, has led to a demand for greater access to critical surface treatments, either through more flexible secondary sources or by transferring surface treatment production onto their own factory floor.
VSi Parylene is simplifying this risk mitigation process by offering forward-thinking products and services that meet the specific needs of the MDM supply environment. For MDMs who require that parts never leave their hands or who want maximum control, VSi provides a stand-alone parylene production system that is easily transferable. This includes optional installation, staff training, as well as process setup and support. This can help customers establish a qualified process that produces high-quality film coatings according to their unique specifications. For MDMs who need a phased transition, flexible hybrid coating services allow customers to begin by outsourcing parylene coating at first and later bring the process in-house.
How to Select a Parylene Supply Partner
You want a provider with a flexible business model who can meet the needs of your business now and in the future. This provider should display a deep understanding of the medical device manufacturing and development process. If they do, they will likely have a quality management system that is International Organization of Standardization (ISO) 13485 certified to help streamline parylene integration and avoid costly headaches on the road to FDA approval. Lastly, select a vendor who values flexibility, expertise, and transparency to ensure what you create together will produce better, more customized results for your specific application.Stefano Longo is a product manager at VSi Parylene, a provider of parylene coatings and innovative technology solutions. He believes parylene has been underutilized in multiple industries and could be the key to unlocking the next milestone in your product’s lifecycle. Functioning as the junction between product management, applications engineering, and customer service, Longo helps consult customers on how parylene can benefit their device and provide longer lifespans to mission-critical devices. He holds a BA in environmental science and geology, and is currently working on a Master of Business Administration, both from the University of Colorado. Previous work experience include positions within the renewable and finance sectors.