Sam Brusco, Associate Editor05.02.22
It’s been a productive last few years for diabetes management technology.
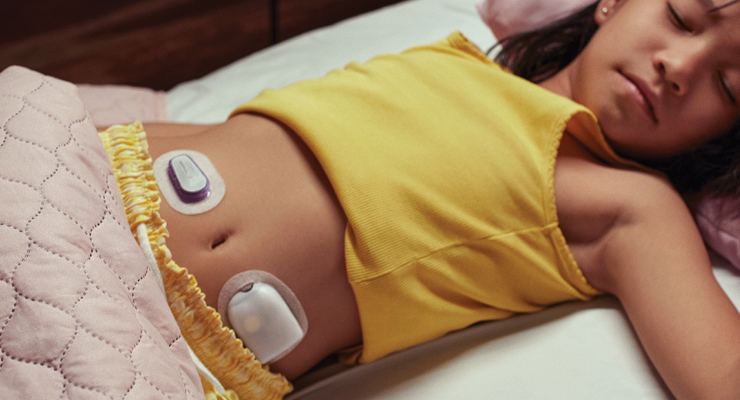
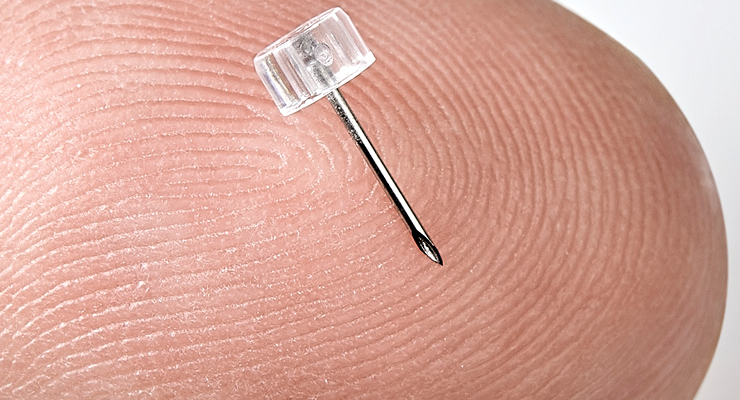
Diabetes patients previously had to get used to pricking their fingers several times a day to track their blood glucose levels. But the continuous glucose monitor (CGM) has offered patients enormous relief. A CGM still requires the sensor to be inserted with a brief needle poke when it’s placed, but today’s sensors can be worn for weeks or even months. Plus, they’re so small patients may often forget they have them on the body.
Insulin pumps were also pretty complicated in the past, often cumbersome and impeding daily activities. But now the devices have become small enough to work with patients’ daily lives, with some delivering continuous and customized doses of rapid-acting insulin 24 hours a day to keep up with the body’s needs. Modern pumps can provide insulin in two ways: basal insulin, small amounts released continuously throughout the day, and bolus insulin, which can be delivered on demand to match food intake or resolve high blood sugar. The pump delivers insulin through a thin flexible tube called an infusion set.
“Diabetes technology is all about making patients’ lives as tolerable as possible while simultaneously improving outcomes,” Dr. Francine Kaufman, chief medical officer of Senseonics, a Germantown, Md.-based long-term implantable CGM maker, told MPO. “This means patients and healthcare providers are looking for tools to help patients manage their condition more effectively while they live their lives however they choose. People want the choice, functionality, and interoperability that allows them to seamlessly integrate technology to meet their condition’s unique needs.”
Unfortunately as high-tech diabetes tech innovations improve management of the chronic disease, patients in underserved communities may often lag behind in making the most of insulin pumps and CGMs. Disparities in race, education, income, and location can prevent some patients with diabetes from receiving necessary care. The medical community has made many attempts to make these essential tools available for everyone, and lawmakers have made some headway to prioritize equitable advancements in research, detection, and care.
“We strive to make Pod therapy universally and globally accessible to everyone requiring daily insulin,” Chuck Alpuche, EVP and COO of Insulet, an Acton, Mass.-based insulin delivery device maker, told MPO. “We work with policymakers and health insurance plans to expand coverage and also secure coverage through the pharmacy channel to offer Omnipod with no up-front cost and no commitment. Our 30-day trial program allows users to experience Omnipod without commitment and we provide financial assistance for those who can’t afford Omnipod (U.S. only).”
Further, using digital technologies can enable safe, effective remote care for diabetes patients. Digital technologies can’t replace standard care of course, but they can enhance existing care pathways by allowing some virtual appointments or providing near-real time monitoring of the patient’s condition. Remote patient monitoring was a crucial part of care delivery during the COVID-19 pandemic and continues to be, but it’s use doesn’t start and end there. It has the potential to boost or fundamentally change healthcare pathways, improve efficiency, and help deliver better outcomes.
“We aim to meet people where they are by making real-time adjustments for the world we live in,” Ali Dianaty, VP of product innovation and operations for global medical device maker Medtronic, told MPO. “For many years, we’ve had our CareLink software to connect data between a patient’s pump and their healthcare team. The COVID-19 pandemic illustrated how important this remote connection could be—when people couldn’t visit their doctor and needed a virtual appointment, our software could share important diabetes data and retain continuity of care.”
San Diego-based continuous glucose monitor (CGM) manufacturer Dexcom’s G6 iCGM (integrated continuous glucose monitoring) system was first authorized through de novo marketing clearance for diabetes patients aged 2 and older in 2018. It was then the first type of CGM authorized by the FDA to be used as part of an integrated system with other medical devices and compatible interfaces—automated insulin dosing systems, insulin pumps, blood glucose meters, or other partner technologies for diabetes management. The G6 Pro, which gathers real-time glucose data over 10 days and offers both blinded and unblinded modes, obtained an FDA nod in 2019.
Further, G6 received FDA breakthrough status in March for use in the hospital setting, providing a more streamlined and efficient review pathway.
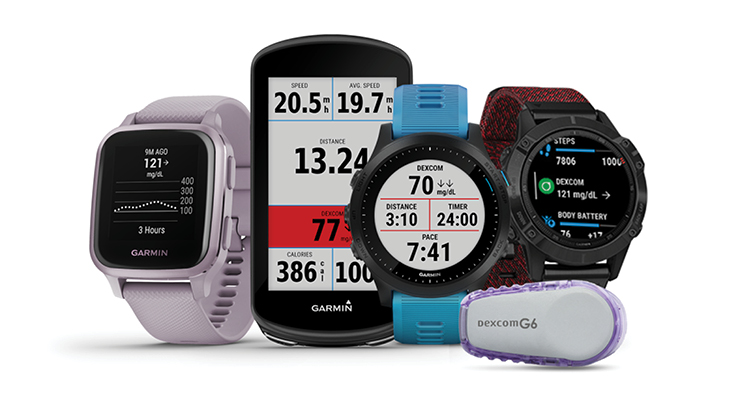
“Dexcom G6 is the most connected CGM on the market, integrating with insulin pens, pumps (automated insulin delivery systems), and digital health apps like Sugarmate, Glooko, and Garmin,” Dexcom CEO Kevin Sayer told MPO.
G6 paired with Sugarmate gives CGM users a phone call when glucose levels are below normal while asleep, texts friends or family if the value drops too low, and pings customizable alerts when levels climb, fall, or remain steady but high. With Glooko, G6 users can be remotely monitored, have access to data insights and population management features, and upload data. Via the Dexcom Connect IQ apps (developed by Garmin) patients can quickly view glucose levels and trends on a compatible Garmin smartwatch or cycling computer, even while exercising.
“We actively look for opportunities to partner with companies that manufacture pumps, pens, and other digital health apps and services to enhance our product and overall user experience,” said Sayer. “The latest examples include Dexcom G6’s integration with hybrid closed-loop systems like Tandem Control-IQ and the Omnipod 5 System, the latter of which received FDA clearance earlier this year.”
An FDA submission for Dexcom’s latest iteration, G7, was made in Q4 of last year. G7 accuracy and safety data for both type 1 and 2 diabetes was published in Diabetes Technology & Therapeutics this year, demonstrating mean absolute relative difference (MARD) of 8.2 percent for adults using arm-placed sensors and showed excellent accuracy independent of diabetes type or treatment regimen.
G7 earned a CE mark in March for European diabetes patients aged 2 and older, including pregnant women.
“Our next-generation Dexcom G7 device takes everything users love about Dexcom G6 and makes it better,” said Sayer. “The wearable will be 60 percent smaller in size, include an intuitive, all-in-one app experience, enhanced alerts, and an easy-to-use optional receiver—people with diabetes and HCPs all highly anticipate these features.”
This past January, Insulet earned FDA clearance for the latest generation of Omnipod, the Omnipod 5 automated insulin delivery (AID) system. The waterproof, tubeless insulin Pod can be worn almost anywhere the user would inject insulin. Cleared for type 1 diabetes patients aged 6 years and above, Omnipod 5 can be controlled with a compatible smartphone. It also integrates with Dexcom’s G6 CGM to help protect against blood glucose highs and lows.
Each Pod AID system allows users to trade multiple daily insulin injections for up to 72 hours of continuous insulin—combined with new automated and CGM integrated features.
“Everything about the Omnipod 5 was designed to be as streamlined and simple as possible to reduce effort, stress, and cognitive burden,” said Alpuche. “In automated mode, it automatically adjusts insulin delivery to protect against highs and lows, day and night. The sophisticated, tubeless, wearable Pod is enhanced with SmartAdjust technology to continuously adjust insulin using a customizable glucose target. It can be fully controlled by a compatible smartphone with no multiple daily injections or tubes, and zero fingersticks.”
Shortly after Omnipod 5’s clearance, Insulet completed an FDA submission to expand its indication to age 2 and up and anticipates being granted the expanded indication later this year. The company also recently finished a type 2 feasibility study that was shared at this April’s Advanced Technologies & Treatments for Diabetes (ATTD) conference. Insulet will take the results from this study to design its pivotal study in order to expand Omnipod 5 to the type 2 diabetes community.
“In the wake of Omnipod 5’s launch, we continue to strategically invest in a robust product pipeline,” said Alpuche. “You’ll see us advance this platform in the coming years—our iOS app development is making terrific progress, as is our integration work with Dexcom’s G7 and Abbott’s FreeStyle Libre.”
The latest iteration of Germantown, Md.-based long-term implantable continuous glucose monitor (CGM) maker Senseonics’ Eversense CGM, the E3, achieved FDA approval in February. The first patient implantation and subsequent launch took place in early April. The E3 features a fully implantable fluorescence-based sensor and removable smart transmitter that provides on-body discreet vibration alerts and transmits glucose level data to a mobile app.
Eversense E3 requires fewer calibrations compared to previous systems, though fingerstick blood glucose measurements are still needed to calibrate it, when symptoms don’t match CGM info, or when taking tetracycline medications.
“The key difference about the next-generation Eversense E3 system is its sensor includes a sacrificial boronic acid (SBA) design modification to enhance sensor survival for up to six months—essentially doubling its lifetime,” said Dr. Kaufman. “This will make it the longest lasting CGM system available, with essentially only two sensor replacements required each year. People with diabetes will be able to break free from some aspects of traditional CGM use like regular sensor changes and ongoing ordering of supplies.”
Senseonics reported results from its PROMISE study last year, which demonstrated excellent accuracy of the new system at six months. The company’s Eversense NOW remote monitoring app also achieved CE mark approval on Android operating systems so users can share data with chosen friends and family members. The company aims to launch the app in Europe soon.
Medtronic’s latest insulin pump innovation in Europe was the advanced hybrid closed-loop system, which automatically adjusts insulin delivery based on readings from the integrated CGM and automates basal and bolus insulin every five minutes. It leverages the company’s MiniMed 780G system and Guardian Sensor 3.
“One of the hardest elements for many people living with diabetes is carb counting at meals. We focused on relieving burden around this in the new system,” said Dianaty. “If a person miscalculates carbs, that’s OK—the pump system will spot the problem and automatically adjust to provide the appropriate amount of insulin.”
Last October Medtronic released one-year, real-world clinical data for 3,211 type 1 diabetes patients aged 15 and below using the MiniMed 780G system with the Guardian Sensor 3 CGM; data showed the advanced hybrid closed-loop algorithm offered stronger glycemic control and protection against lows while sleeping (average time in range 77 percent; overnight time in range 82 percent).
According to the company, the next-gen MiniMed 780G system with Guardian 4 Sensor is under active FDA review.
“In the future, we aim to further personalize the automation of diabetes systems. We are working to leverage the power of artificial intelligence to do a lot of the manual work for people using this technology,” said Dianaty. “For example, gesture technology could help identify when food is being consumed by the hand motion a smartwatch can detect. That could be linked to diabetes tech to reduce the number of engagements or touchpoints needed to optimize therapy.
This can be coupled with predictive analytic capabilities around mealtimes to help identify what’s being consumed with greater accuracy. People react to food in different ways, so these AI advancements with personalization could change the ease of managing diabetes.”
Advanced diabetes technology was further expanded in December when the U.S. Centers for Medicare & Medicaid Services (CMS) issued a ruling to expand Medicare coverage for all CGMs, both adjunctive and non-adjunctive. This included CGMs that integrate with Medtronic’s insulin pumps. The ruling became effective in mid-February.
CGMs and insulin pumps contain a number of specialized components to accomplish their lifesaving work. Most CGMs contain a monitor to display the information—in some cases, the patient’s mobile device—a sensor usually inserted into the subcutaneous tissue, and a transmitter that sends sensor data to the monitor.
An insulin pump is also typically made up of four main parts. One is a small cartridge of insulin called a reservoir. A thin plastic tube called a cannula sits under the skin. Flexible plastic tubing connects the cannula to the reservoir. And a pump allows patients to set and change the amount of insulin delivered.
Some of these components are quite small, especially with diabetes management devices’ need to be discreet and not impede the wearer’s daily life. Making these components requires the ability to manufacture parts with form factors and tolerances that traditional fabrication methods will not be able to accomplish. For example, some components going into CGMs or insulin pumps require the services of a micromolder who can create these tiny parts.
“Micromolded components used in diabetes and glucose monitoring are cannulas, sheaths, capillary action fluidic components, pumps, gears, flow restrictors, flow enablers, and miniature valves, to name a few,” said Donna Bibber, CEO of Isometric Micro Molding, a New Richmond, Wis.-based medical-focused micro molder.
Components like sheaths and cannulas going into diabetes management devices can stretch the limits of manufacturing possibility. These sheaths and cannulas can require extremely thin wall configurations. Some micromolders are able to accomplish this very specialized and precise type of manufacturing. According to Isometric Micro Molding, resin companies have said there’s a limit to the flow length (thickness), but the company has been able to surpass flow lengths and limits.
“Designing products with multiple part assemblies requires the partnership with a micromolder and preferably one that is also an automated assembler,” said Bibber. “With 16 cavities of one part, 32 cavities of another, and eight cavities of yet another, all the full factorial combinations of each mold parts’ cavities assembled error must equate to at least 1.33 Cpk or better. The assembly is only as good as the weakest Cpk capable component. Isometric Micro Molding works in tight tolerances including single microns all day, every day and has the mindset and over 30 years of experience to solve problems arising from these challenges. The part may not be micro in size, but the diabetes device’s precision may also require a micromolder’s and automated micro assembler’s skills.”
The technological innovations around CGMs and insulin pumps have challenged manufacturing partners involved in all facets of the devices’ manufacture.
“We’re asking polymers to accomplish things they haven’t before,” said Bill Torris, director of medical solutions’ Technical Design Center in North America for Nolato, a developer and producer of polymer-based products. “In previous devices, a needle was glued in place to a piece of plastic, now there’s a wire going under the skin and into the device. This device has power and an antenna so there can’t be liquids present and it has to be sealed. To do that, you have to transmit electricity—plastics are terrible for that because they’re an insulator. So we design and compound silicones and use other polymers that can both conceal and transmit electricity. Part of making a device smaller means getting its components inside to do multiple things.”
“For example, what may have been a standard rubber O-ring was only sealing,” he continued. “Two or three O-rings may be needed to properly seal the device. You have to consider attachment methods and whether sealing is its only function. You have to also be aware of how much real estate the component takes up.”
A shrinking form factor for CGMs and insulin pumps means the electronics are smaller and more tightly packed together, yet another challenge for manufacturing partners for these devices.
“When molding silicone around a battery for a diabetes device, for example, it needs to be sealed but also conduct the electricity to power the device,” said Torris. “We have to explore what kind of methods to manufacture the polymer and also worry about getting the electrical signal out. This could involve laser ablation, plating a rigid plastic, and putting additives in the polymer to conduct electricity.”
“We have the ability to formulate our own silicones—we’re one of a handful of companies that provides these proprietary materials that need to be used in many diabetes management products,” Torris went on. We can intentionally put additives in to ensure they do or don’t conduct electricity, offset those materials, and start to accomplish what’s necessary. That can either be adding thermal shielding properties or making it more thermally conductive—you’re trying to take heat away from one part of the device and put it into a different part. We do EMC shielding as well because there are more and more electronics in these devices.”
As diabetes management technologies continue to evolve, manufacturing partners will be further challenged to innovate their own processes alongside the device makers.
“The capability of molders for tooling is being challenged, and that’s where manufacturing simulation comes in,” said Torris. “Using this you can design a chassis with the fluid path, and can custom create a polymer that’s going to seal and/or conduct to thermally shield or wick away. The need for smaller wearables is driving some components toward micromolding because the process is beneficial for insulin delivery components and much more in the medical field.”
Diabetes patients previously had to get used to pricking their fingers several times a day to track their blood glucose levels. But the continuous glucose monitor (CGM) has offered patients enormous relief. A CGM still requires the sensor to be inserted with a brief needle poke when it’s placed, but today’s sensors can be worn for weeks or even months. Plus, they’re so small patients may often forget they have them on the body.
Insulin pumps were also pretty complicated in the past, often cumbersome and impeding daily activities. But now the devices have become small enough to work with patients’ daily lives, with some delivering continuous and customized doses of rapid-acting insulin 24 hours a day to keep up with the body’s needs. Modern pumps can provide insulin in two ways: basal insulin, small amounts released continuously throughout the day, and bolus insulin, which can be delivered on demand to match food intake or resolve high blood sugar. The pump delivers insulin through a thin flexible tube called an infusion set.
“Diabetes technology is all about making patients’ lives as tolerable as possible while simultaneously improving outcomes,” Dr. Francine Kaufman, chief medical officer of Senseonics, a Germantown, Md.-based long-term implantable CGM maker, told MPO. “This means patients and healthcare providers are looking for tools to help patients manage their condition more effectively while they live their lives however they choose. People want the choice, functionality, and interoperability that allows them to seamlessly integrate technology to meet their condition’s unique needs.”
Unfortunately as high-tech diabetes tech innovations improve management of the chronic disease, patients in underserved communities may often lag behind in making the most of insulin pumps and CGMs. Disparities in race, education, income, and location can prevent some patients with diabetes from receiving necessary care. The medical community has made many attempts to make these essential tools available for everyone, and lawmakers have made some headway to prioritize equitable advancements in research, detection, and care.
“We strive to make Pod therapy universally and globally accessible to everyone requiring daily insulin,” Chuck Alpuche, EVP and COO of Insulet, an Acton, Mass.-based insulin delivery device maker, told MPO. “We work with policymakers and health insurance plans to expand coverage and also secure coverage through the pharmacy channel to offer Omnipod with no up-front cost and no commitment. Our 30-day trial program allows users to experience Omnipod without commitment and we provide financial assistance for those who can’t afford Omnipod (U.S. only).”
Further, using digital technologies can enable safe, effective remote care for diabetes patients. Digital technologies can’t replace standard care of course, but they can enhance existing care pathways by allowing some virtual appointments or providing near-real time monitoring of the patient’s condition. Remote patient monitoring was a crucial part of care delivery during the COVID-19 pandemic and continues to be, but it’s use doesn’t start and end there. It has the potential to boost or fundamentally change healthcare pathways, improve efficiency, and help deliver better outcomes.
“We aim to meet people where they are by making real-time adjustments for the world we live in,” Ali Dianaty, VP of product innovation and operations for global medical device maker Medtronic, told MPO. “For many years, we’ve had our CareLink software to connect data between a patient’s pump and their healthcare team. The COVID-19 pandemic illustrated how important this remote connection could be—when people couldn’t visit their doctor and needed a virtual appointment, our software could share important diabetes data and retain continuity of care.”
San Diego-based continuous glucose monitor (CGM) manufacturer Dexcom’s G6 iCGM (integrated continuous glucose monitoring) system was first authorized through de novo marketing clearance for diabetes patients aged 2 and older in 2018. It was then the first type of CGM authorized by the FDA to be used as part of an integrated system with other medical devices and compatible interfaces—automated insulin dosing systems, insulin pumps, blood glucose meters, or other partner technologies for diabetes management. The G6 Pro, which gathers real-time glucose data over 10 days and offers both blinded and unblinded modes, obtained an FDA nod in 2019.
Further, G6 received FDA breakthrough status in March for use in the hospital setting, providing a more streamlined and efficient review pathway.
“Dexcom G6 is the most connected CGM on the market, integrating with insulin pens, pumps (automated insulin delivery systems), and digital health apps like Sugarmate, Glooko, and Garmin,” Dexcom CEO Kevin Sayer told MPO.
G6 paired with Sugarmate gives CGM users a phone call when glucose levels are below normal while asleep, texts friends or family if the value drops too low, and pings customizable alerts when levels climb, fall, or remain steady but high. With Glooko, G6 users can be remotely monitored, have access to data insights and population management features, and upload data. Via the Dexcom Connect IQ apps (developed by Garmin) patients can quickly view glucose levels and trends on a compatible Garmin smartwatch or cycling computer, even while exercising.
“We actively look for opportunities to partner with companies that manufacture pumps, pens, and other digital health apps and services to enhance our product and overall user experience,” said Sayer. “The latest examples include Dexcom G6’s integration with hybrid closed-loop systems like Tandem Control-IQ and the Omnipod 5 System, the latter of which received FDA clearance earlier this year.”
An FDA submission for Dexcom’s latest iteration, G7, was made in Q4 of last year. G7 accuracy and safety data for both type 1 and 2 diabetes was published in Diabetes Technology & Therapeutics this year, demonstrating mean absolute relative difference (MARD) of 8.2 percent for adults using arm-placed sensors and showed excellent accuracy independent of diabetes type or treatment regimen.
G7 earned a CE mark in March for European diabetes patients aged 2 and older, including pregnant women.
“Our next-generation Dexcom G7 device takes everything users love about Dexcom G6 and makes it better,” said Sayer. “The wearable will be 60 percent smaller in size, include an intuitive, all-in-one app experience, enhanced alerts, and an easy-to-use optional receiver—people with diabetes and HCPs all highly anticipate these features.”
This past January, Insulet earned FDA clearance for the latest generation of Omnipod, the Omnipod 5 automated insulin delivery (AID) system. The waterproof, tubeless insulin Pod can be worn almost anywhere the user would inject insulin. Cleared for type 1 diabetes patients aged 6 years and above, Omnipod 5 can be controlled with a compatible smartphone. It also integrates with Dexcom’s G6 CGM to help protect against blood glucose highs and lows.
Each Pod AID system allows users to trade multiple daily insulin injections for up to 72 hours of continuous insulin—combined with new automated and CGM integrated features.
“Everything about the Omnipod 5 was designed to be as streamlined and simple as possible to reduce effort, stress, and cognitive burden,” said Alpuche. “In automated mode, it automatically adjusts insulin delivery to protect against highs and lows, day and night. The sophisticated, tubeless, wearable Pod is enhanced with SmartAdjust technology to continuously adjust insulin using a customizable glucose target. It can be fully controlled by a compatible smartphone with no multiple daily injections or tubes, and zero fingersticks.”
Shortly after Omnipod 5’s clearance, Insulet completed an FDA submission to expand its indication to age 2 and up and anticipates being granted the expanded indication later this year. The company also recently finished a type 2 feasibility study that was shared at this April’s Advanced Technologies & Treatments for Diabetes (ATTD) conference. Insulet will take the results from this study to design its pivotal study in order to expand Omnipod 5 to the type 2 diabetes community.
“In the wake of Omnipod 5’s launch, we continue to strategically invest in a robust product pipeline,” said Alpuche. “You’ll see us advance this platform in the coming years—our iOS app development is making terrific progress, as is our integration work with Dexcom’s G7 and Abbott’s FreeStyle Libre.”
The latest iteration of Germantown, Md.-based long-term implantable continuous glucose monitor (CGM) maker Senseonics’ Eversense CGM, the E3, achieved FDA approval in February. The first patient implantation and subsequent launch took place in early April. The E3 features a fully implantable fluorescence-based sensor and removable smart transmitter that provides on-body discreet vibration alerts and transmits glucose level data to a mobile app.
Eversense E3 requires fewer calibrations compared to previous systems, though fingerstick blood glucose measurements are still needed to calibrate it, when symptoms don’t match CGM info, or when taking tetracycline medications.
“The key difference about the next-generation Eversense E3 system is its sensor includes a sacrificial boronic acid (SBA) design modification to enhance sensor survival for up to six months—essentially doubling its lifetime,” said Dr. Kaufman. “This will make it the longest lasting CGM system available, with essentially only two sensor replacements required each year. People with diabetes will be able to break free from some aspects of traditional CGM use like regular sensor changes and ongoing ordering of supplies.”
Senseonics reported results from its PROMISE study last year, which demonstrated excellent accuracy of the new system at six months. The company’s Eversense NOW remote monitoring app also achieved CE mark approval on Android operating systems so users can share data with chosen friends and family members. The company aims to launch the app in Europe soon.
Medtronic’s latest insulin pump innovation in Europe was the advanced hybrid closed-loop system, which automatically adjusts insulin delivery based on readings from the integrated CGM and automates basal and bolus insulin every five minutes. It leverages the company’s MiniMed 780G system and Guardian Sensor 3.
“One of the hardest elements for many people living with diabetes is carb counting at meals. We focused on relieving burden around this in the new system,” said Dianaty. “If a person miscalculates carbs, that’s OK—the pump system will spot the problem and automatically adjust to provide the appropriate amount of insulin.”
Last October Medtronic released one-year, real-world clinical data for 3,211 type 1 diabetes patients aged 15 and below using the MiniMed 780G system with the Guardian Sensor 3 CGM; data showed the advanced hybrid closed-loop algorithm offered stronger glycemic control and protection against lows while sleeping (average time in range 77 percent; overnight time in range 82 percent).
According to the company, the next-gen MiniMed 780G system with Guardian 4 Sensor is under active FDA review.
“In the future, we aim to further personalize the automation of diabetes systems. We are working to leverage the power of artificial intelligence to do a lot of the manual work for people using this technology,” said Dianaty. “For example, gesture technology could help identify when food is being consumed by the hand motion a smartwatch can detect. That could be linked to diabetes tech to reduce the number of engagements or touchpoints needed to optimize therapy.
This can be coupled with predictive analytic capabilities around mealtimes to help identify what’s being consumed with greater accuracy. People react to food in different ways, so these AI advancements with personalization could change the ease of managing diabetes.”
Advanced diabetes technology was further expanded in December when the U.S. Centers for Medicare & Medicaid Services (CMS) issued a ruling to expand Medicare coverage for all CGMs, both adjunctive and non-adjunctive. This included CGMs that integrate with Medtronic’s insulin pumps. The ruling became effective in mid-February.
CGMs and insulin pumps contain a number of specialized components to accomplish their lifesaving work. Most CGMs contain a monitor to display the information—in some cases, the patient’s mobile device—a sensor usually inserted into the subcutaneous tissue, and a transmitter that sends sensor data to the monitor.
An insulin pump is also typically made up of four main parts. One is a small cartridge of insulin called a reservoir. A thin plastic tube called a cannula sits under the skin. Flexible plastic tubing connects the cannula to the reservoir. And a pump allows patients to set and change the amount of insulin delivered.
Some of these components are quite small, especially with diabetes management devices’ need to be discreet and not impede the wearer’s daily life. Making these components requires the ability to manufacture parts with form factors and tolerances that traditional fabrication methods will not be able to accomplish. For example, some components going into CGMs or insulin pumps require the services of a micromolder who can create these tiny parts.
“Micromolded components used in diabetes and glucose monitoring are cannulas, sheaths, capillary action fluidic components, pumps, gears, flow restrictors, flow enablers, and miniature valves, to name a few,” said Donna Bibber, CEO of Isometric Micro Molding, a New Richmond, Wis.-based medical-focused micro molder.
Components like sheaths and cannulas going into diabetes management devices can stretch the limits of manufacturing possibility. These sheaths and cannulas can require extremely thin wall configurations. Some micromolders are able to accomplish this very specialized and precise type of manufacturing. According to Isometric Micro Molding, resin companies have said there’s a limit to the flow length (thickness), but the company has been able to surpass flow lengths and limits.
“Designing products with multiple part assemblies requires the partnership with a micromolder and preferably one that is also an automated assembler,” said Bibber. “With 16 cavities of one part, 32 cavities of another, and eight cavities of yet another, all the full factorial combinations of each mold parts’ cavities assembled error must equate to at least 1.33 Cpk or better. The assembly is only as good as the weakest Cpk capable component. Isometric Micro Molding works in tight tolerances including single microns all day, every day and has the mindset and over 30 years of experience to solve problems arising from these challenges. The part may not be micro in size, but the diabetes device’s precision may also require a micromolder’s and automated micro assembler’s skills.”
The technological innovations around CGMs and insulin pumps have challenged manufacturing partners involved in all facets of the devices’ manufacture.
“We’re asking polymers to accomplish things they haven’t before,” said Bill Torris, director of medical solutions’ Technical Design Center in North America for Nolato, a developer and producer of polymer-based products. “In previous devices, a needle was glued in place to a piece of plastic, now there’s a wire going under the skin and into the device. This device has power and an antenna so there can’t be liquids present and it has to be sealed. To do that, you have to transmit electricity—plastics are terrible for that because they’re an insulator. So we design and compound silicones and use other polymers that can both conceal and transmit electricity. Part of making a device smaller means getting its components inside to do multiple things.”
“For example, what may have been a standard rubber O-ring was only sealing,” he continued. “Two or three O-rings may be needed to properly seal the device. You have to consider attachment methods and whether sealing is its only function. You have to also be aware of how much real estate the component takes up.”
A shrinking form factor for CGMs and insulin pumps means the electronics are smaller and more tightly packed together, yet another challenge for manufacturing partners for these devices.
“When molding silicone around a battery for a diabetes device, for example, it needs to be sealed but also conduct the electricity to power the device,” said Torris. “We have to explore what kind of methods to manufacture the polymer and also worry about getting the electrical signal out. This could involve laser ablation, plating a rigid plastic, and putting additives in the polymer to conduct electricity.”
“We have the ability to formulate our own silicones—we’re one of a handful of companies that provides these proprietary materials that need to be used in many diabetes management products,” Torris went on. We can intentionally put additives in to ensure they do or don’t conduct electricity, offset those materials, and start to accomplish what’s necessary. That can either be adding thermal shielding properties or making it more thermally conductive—you’re trying to take heat away from one part of the device and put it into a different part. We do EMC shielding as well because there are more and more electronics in these devices.”
As diabetes management technologies continue to evolve, manufacturing partners will be further challenged to innovate their own processes alongside the device makers.
“The capability of molders for tooling is being challenged, and that’s where manufacturing simulation comes in,” said Torris. “Using this you can design a chassis with the fluid path, and can custom create a polymer that’s going to seal and/or conduct to thermally shield or wick away. The need for smaller wearables is driving some components toward micromolding because the process is beneficial for insulin delivery components and much more in the medical field.”