Michael Barbella, Managing Editor06.02.21
Ask and ye shall receive.
Sometimes, innovations are just that simple.
Consider the endoscope, for instance. Aside from a few rudimentary peeks, interior portions of the esophageal tract, stomach, and intestines were largely unexplored territories for several millennia, their function and foibles relegated mainly to imagination and conjecture.
Those realms would have remained uncharted, too, had it not been for a very basic request.
That appeal came from Tatsuro Uji, M.D., a physician at Tokyo University Hospital in Koishikawa. The year was 1949, and Uji—like many of his colleagues—was concerned about rising stomach cancer rates in Japan. Although tools had been developed over the previous 143 years to look inside the human body (including one that used a candle and angled mirrors), none could actually capture images.
Uji turned to Olympus Optical Co. Ltd. (now Olympus Corporation) for help in developing a camera that could photograph and examine the interior of a patient’s stomach. Mutsuo Sugiura, an Olympus engineer who was the same age as Uji, welcomed the request, reportedly saying, “As long as there is light, a lens, and a film, a camera can take pictures anywhere.”
Maybe so, but taking pictures inside the human body can be particularly challenging. Besides being safe for patients, internal body cameras also must comfortably navigate the gastrointestinal tract, quickly record snapshots, and produce sharp images for easier diagnosis. To achieve these objectives, the “gastrocamera” would require extremely small lenses, strong illumination sources, flexible materials (for tubes), and water leakage prevention capabilities.
“How to make an ultra-small camera that could be inserted into the stomach and still take clear photographs,” Toshio Nakatsubo, a former Olympus Corp. employee who contributed to the endoscope’s development, recalled in a 1998 Oyo-Buturi International interview. “That was the challenge. Being 1949, there was very little useful technology available, nor basic materials for construction for us [to] use.”
Despite all the hurdles, Olympus engineers developed a prototype in 1950 that featured a photographic lens located at the tip of a flexible tube, according to company history. Clinicians captured images on monochrome film by manually photoflashing a miniature light bulb in-vitro, and they wound the film by pulling a wire.
After various tweaks (one being a thinner tube), Olympus unveiled its gastrocamera—the GT-1—to great fanfare in late 1950. But the devices fared poorly in real clinical settings, often malfunctioning during photo sessions. Doctors soon inundated the company with product returns and repair requests.
While discouraging, the faulty products actually helped spawn advancements that eventually transformed the gastrocamera into an indispensable diagnostic tool. One such improvement was the incorporation of fiber optics (1964), which allowed doctors to examine the stomach in real time. Equally significant was the development of a video monitor system for simultaneously viewing those real-time images.
With subsequent advancements—transducers, HDTV, narrow-band imaging, self-navigation, autofluorescence, shape-locking overtubes, and light-scattering spectroscopy (among others)—the endoscope has proven its value to healthcare systems worldwide.
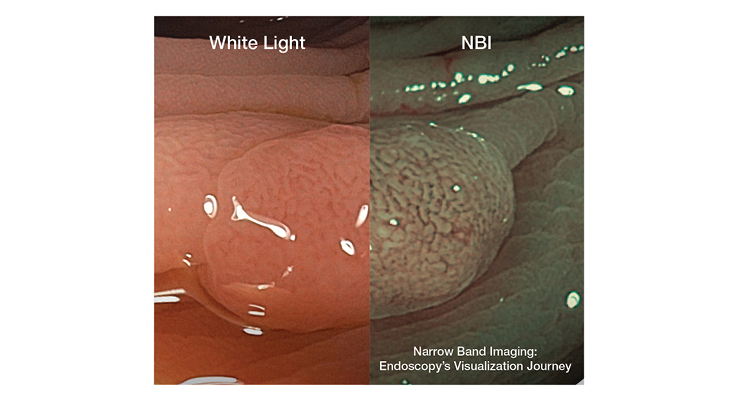
“If you go back to the dawn of endoscopy, it basically amounted to sticking a film camera at the end of a long tube in the stomach, snapping pictures blindly, and seeing what you get,” said Philip Doyle, executive director of Endoscopy Sales & Marketing for Olympus America Inc. “The technology has come a long way since then. After the original gastrocamera came fiber optic scopes, where you hold your eye to the top of the scope, you look through the fiber optic bundle, and you’re seeing in real-time what the scope is pointing at. From there, we went to video endoscopy, which was even better, and we progressed from standard to high-definition resolution. The resolution power of endoscopes has become better over time, we can see a lot more fine details and that’s helpful because some lesions may not be so obvious. Now you are better able to see what you otherwise could have overlooked with lesser image quality from the past. Narrow band imaging (NBI) helps to enhance the visibility of blood vessels, it filters white light into specific wavelengths that interact with hemoglobin and provides contrast for superficial blood vessels on the mucosal surface. It helps give physicians a better chance of detecting abnormal tissue and a better understanding of what they have found even before a pathologist gets back to them.”
Indeed, in the six decades since its birth, the endoscope has become a cornerstone of clinical treatment, begetting a multi-billion-dollar global market that is forecast to swell 8 percent annually over the next seven years, industry data indicate. Familiar market drivers are fueling the expansion: rising chronic disease caseloads (i.e., cancer, diabetes, heart conditions), as well as the increasing prevalence of (and preference for) minimally invasive (MI) surgery.
“There are a few major trends shaping the global endoscopic procedures market. The first is the broadly increasing adoption rate of minimally invasive or endoscopic procedures within the surgical setting,” said Charles Golub, market development manager at Saint-Gobain Life Sciences Medical Components, a Northborough, Mass.-based contract manufacturer focused on customized material solutions from silicone, thermoplastic elastomers, and polyvinyl chloride. “Previously, standard treatment options involved highly invasive procedures; recently though, endoscopic procedures are becoming more routine, which is enabling quicker patient recovery times and reducing the burden on hospitals.”
It’s also enabling new product innovations. Last fall, Olympus introduced two U.S. Food and Drug Administration (FDA)-cleared colonoscopes that expand the company’s capabilities in MI procedures, including endoscopic submucosal dissection (ESD).
The PCF-H190T endoscope is a slimmer, short-bending, high-definition device made for turning tight corners. Designed to help classify colorectal polyps, the PCF-H190T features better retroflexion capability than its predecessors (the technique is commonly used to detect distal rectal polyps), and a channel layout appropriate for passing through therapeutic accessories, such as for hemostasis or polypectomy. The device also is inserted more easily into the rectum, for better patient comfort.
Both the PCF-H190T and its sister product, the PCF-HQ190, are used with the EVIS EXERA III imaging platform. The PCF-HQ190 contains dual-focus hardware for visualizing the intestine’s mucus membranes in a 170-degree field-of-view. The scope’s NBI and its near focus mode have a greater than 90 percent agreement with pathological analysis in assigning post-polypectomy surveillance after a colonoscopy, according to Olympus.
The PCF-HQ190 also features Responsive Insertion Technology to facilitate complete colonoscopies and reduced cecal intubation times through better maneuverability. Its built-in ScopeGuide Function provides real-time 3D visualization of the device’s position and configuration during insertion, including identifying scope loops as they form.
“There are many minimally invasive interventions that can be done via endoscopy. These procedures tend to involve less trauma to the patient, decreased recovery time, and lower costs to the healthcare system,” Doyle noted. “Endoscopic ultrasound is one such intervention that comes to mind. It’s a procedure guided by ultrasound, using a special endoscope with an ultrasound probe attached. There’s also endoscopic submucosal dissection, or ESD, and peroral endoscopic myotomy. These kinds of procedures would have required surgical intervention in the past.”
Endobronchial ultrasound would have as well. Sampling mediastinal lymph nodes or peribronchial tumors used to require an invasive chest incision, but endobronchial ultrasound (EBUS) procedures avoid any cuts, relying instead on real-time imaging to access smaller lymph nodes for biopsy.
Two types of EBUS techniques are used for diagnosing lung cancer: radial and transbronchial needle aspiration. Radial EBUS typically produces more accurate nodule samples in accessibly difficult areas while EBUS-TBNA helps clinicians “see” beyond the lungs’ walls.
A Radial EBUS procedure is performed by inserting a miniature ultrasound probe through the working channel of a flexible bronchoscope or catheter (guide sheath), according to Olympus. Real-time imaging of the surrounding tissue enables doctors to determine a lesion’s precise location and size. Upon identification, the probe is withdrawn and a biopsy sampling device is inserted down the scope to collect tissue samples for biopsy.
EBUS-TBNA requires more professional training and is a longer procedure, but usually yields larger samples than conventional TBNA. This approach uses an ultrasound transducer integrated into a flexible fiberoptic bronchoscope for viewing images. Once a satisfactory image is obtained, a biopsy needle with a pre-set stylet length is passed through the bronchoscope’s proximal port, then under direct and real-time vision, is inserted through the endobronchial mucosa and into the lymph node or mass. Three needle aspirations per lymph node is recommended for non-small cell lung cancer screenings.
EBUS-TBNA procedures have correctly staged and diagnosed lung cancer more than 95 percent of the time, and multiple prospective studies have shown a similarly high accuracy rate for detecting extra-thoracic malignancies and lymphomas (85-95 percent and 91-97 percent, respectively).
Olympus and PENTAX Medical are working to boost those scores and improve the EBUS-TBNA technique with newly-launched products designed to enhance endobronchial ultrasound access, visualization, and bronchoscope control.
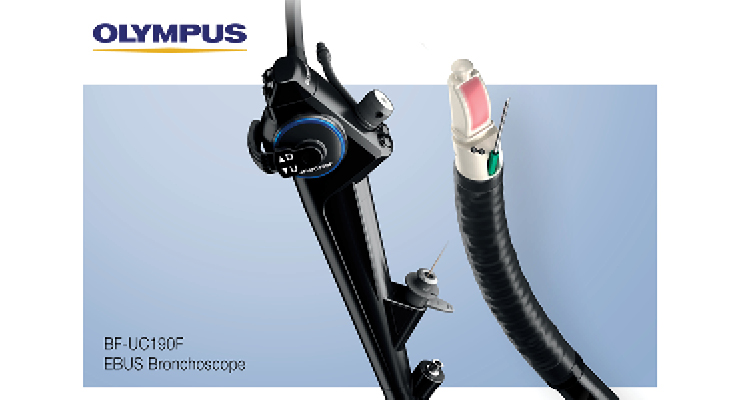
Olympus’ BF-UC190F next-generation EBUS bronchoscope, for example, offers improved lymph node access in hard-to-reach areas and a compact distal tip (6.6mm diameter) for smoother insertion and easier maneuvering in the lung. Launched in May, the device features an increased scope angulation (from 120 degrees to 160 degrees), allowing better positioning of the scope tip relative to the targeted tissue.
Additionally, a more perpendicular needle orientation allows for smoother target penetration between cartilage rings and other critical anatomy, the company claims. The BF-UC190F also generates higher-resolution images, and possesses a decreased forward oblique angle (20 degrees) that keeps the endoscopic image in full field view during airway navigation.
PENTAX Medical’s newest EBUS-TBNA bronchoscope enhances imaging as well. The company claims its EB19-J10U Ultrasound Video Bronchoscope, released last September, provides “crystal clear visualization” of the bronchial anatomy and improved procedural efficiency for tissue sampling.
According to PENTAX, the EB19-J10U is ergonomically designed with a focus on patient comfort, minimum stress on the operator’s hand, and improved endoscopic visibility. Its small, easily maneuverable ultrasound transducer at the bronchoscope’s tip is permanently visible during the procedure, allowing clinicians to confirm its airway positioning. Lung and lymph node imaging is improved with a wide 75-degree field of vision, and a new 2.2-mm working channel is compatible with 19-Gauge needles, a relatively recent development.
“EBUS is recognized as the gold standard for minimally invasive tissue acquisition in pulmonology,” PENTAX Medical Americas President and CEO David Woods said upon the EB19-J10U’s market release. “Combined with Hitachi Ultrasound Scanners, the EB19-J10U reaffirms our commitment to diagnostic and interventional Pulmonology. PENTAX is globally focused on innovations that not only help improve clinical outcomes and reduce the cost of care delivery, but also enhance our customers’ satisfaction. We think the EB19-J10U is a significant building block for this strategy.”
Auris Health Inc. is employing the same strategy, but with a different building block. The company’s Monarch Platform harnesses the proficiencies of robotic technology to better examine the lungs. The platform combines traditional endoscopic views of the body’s airways with computer-assisted navigation based on 3D anatomical models to provide continuous vision during diagnostic or therapeutic bronchoscopy procedures.
The U.S. Food and Drug Administration (FDA)-cleared Monarch system features a video game-style controller that is used to navigate a flexible robotic endoscope to the periphery of the lung with improved reach, vision, and control. The system is designed to more accurately access, diagnose, and treat small, hard-to-reach peripheral lung nodules.
“Conventional tools are limited in their ability to reach and get an early and accurate diagnosis in lung modules,” noted Eric Davidson, president of Flexible Robotics at Auris Health Inc., a company Ethicon Inc., part of the Johnson & Johnson Medical Device Companies, acquired in February 2019 for $3.4 billion. “With Monarch, we are trying to play a role in extending a physician’s capabilities and better define what is possible endoscopically. And we’re starting that journey with lung cancer—specifically, providing bronchoscopic visualization of and access to patient airways for diagnostic and therapeutic procedures. Monarch is designed to overcome the limitations of current diagnostic capabilities by providing visualization and access for the biopsy needle to go where the physician wants it. Lung cancer is a disease that, if caught late, has quite poor survival rates.”
Auris is hopeful the Monarch Platform will eventually boost those rates. An inaugural study of the technology shows promise, with results indicating the system reached target lung lesions in more than 96 percent of patients (52 of 54 people across five sites). Pneumothorax (collapsed lung) occurred in two patients, one of which required the placement of a tube to drain air or fluid from the chest.
The study’s overall diagnostic yield was 74.1 percent, and a 70 percent yield was achieved in lung nodules located outside the patient’s airway. Non-robotic technology reported 30-40 percent diagnostic yields.
“Lung cancer kills more people worldwide than the next three cancers combined. There are 1.5 million lung nodules that present each year in the United States, and they can be deep inside the lung,” Davidson told Medical Product Outsourcing. “That is a very complex area in which to diagnose and treat. With these hard-to-reach nodules, conventional tools make it hard to diagnose lung cancer. For years, there’s been an effort in healthcare to diagnose the disease earlier and more accurately. Monarch is an enabling endoscopic technology—it’s a means to access lung nodules safely and accurately for either diagnostic or therapeutic intent.”
Certainly, the Monarch Platform provides a means to a very specific end, but it’s not the only “enabling” technology of its kind. Natural orifice transluminal endoscopic surgery (NOTES) is becoming a more preferential MI surgical option, as it capitalizes on the body’s natural orifices (mouth, anus, vagina, urethra) to access the abdominal cavity.
NOTES is performed using a flexible endoscope that is advanced into the peritoneal cavity after puncturing an internal organ such as the stomach, colon, vagina, or bladder. Conventional endoscopic instruments are then introduced through the flexible device’s working channels to conduct the procedure.
While NOTES provides all the advantages of laparoscopic surgery, it also offers several added benefits, including lower anesthesia requirements, less pain and scarring, faster recovery, and a fewer incidents of wound-related complications.
“Many endoscopic surgeries started using robotic-assisted devices years ago, specifically some of the thoracic procedures for lung cancer,” Saint Gobain’s Golub stated. “Newer procedures are rapidly adopting this technology, such as the natural orifice (NOTES) procedures. As these devices gain more acceptance and popularity with physicians and practitioners, I anticipate we’ll see many new challenges arise in the design and ease of use for these endoscopic devices.”
Some of those challenges have already surfaced: hysteresis reduction, enhanced maneuverability and degrees of freedom, internal instrument exchange, broader operative capabilities, and part miniaturization.
One of the big challenges with miniaturization is heat dissipation for the LED lighting on the tip of many endoscopes,” said Arthur Roti, general manager of Instrument Technology Inc., a Westfield, Mass.-headquartered designer, developer, and manufacturer of borescopes, fiberscopes, and videoscopes.
Researchers are working fervently to overcome such barriers as interest in gastric applications for NOTES builds among surgeons and advanced endoscopists.
EndoMaster Pte. Ltd., for example, is working to develop an endoscopic robotic system that removes cancerous gastric and intestinal tumors without incisions. The Singapore firm’s flexible endoscopic robot—EndoMaster EASE (Endoluminal Access Surgical Efficacy) System—consists of a flexible platform and endoscopic imaging system that allows the passage of two minute robotic arms to achieve tissue retraction and dissection in ESD (endoscopic submucosal dissection) procedures, according to the Chinese University of Hong Kong (CU Medicine).
The university used the EndoMaster EASE last spring to conduct the world’s first clinical trial on robotic colorectal ESD, an advanced, less invasive endoscopic treatment for early gastrointestinal cancer. Six patients underwent colorectal ESD with the EASE System and no instances of perforation were recorded. Patients resumed a normal diet after one day and were discharged after two days, CU Medicine reported.
“From our initial observation, the flexible endoscopic robotic system is a very promising armamentarium for performing ESD and treating early-stage colorectal cancer,” said Professor Philip Wai Yan Chiu, director of the university’s Jockey Club Minimally Invasive Surgical Skills Center. “Our clinical trial is still currently underway and we will continue to enroll suitable patients to participate.”
The EndoMaster EASE System is neither CE Marked nor FDA approved.
The Hominis platform is off-limits in Europe, too, but is blessed by the FDA for natural orifice laparoscopic-assisted transvaginal benign surgical procedures, including benign hysterectomy. Developed by Tel Aviv, Israel-based Memic Innovative Surgery Ltd., Hominis is designed to remove the uterus using minimally invasive surgical instruments inserted through the vagina, as well as a video camera inserted laparoscopically through a small incision on the abdomen.
The Hominis (Latin for humanoid) system is touted as the “first and only” FDA-authorized surgical robotic platform featuring miniature humanoid-shaped robotic arms that provide human-level dexterity, multi-planar flexibility, and 360 degrees of articulation. The system’s biomimetic instruments aim to replicate the motions and capabilities of a surgeon’s arms, with shoulder, elbow, and wrist joints. Multiple instruments can be introduced to the body through a single portal and the 360-degree articulation offers obstacle avoidance as well as optimal access and working angles.
“We are pleased to receive FDA De Novo authorization of our Hominis system, which offers a small, cost-effective and less invasive option over current robotic instruments limited to straight shaft and single wrist designs and controlled with large, complex and expensive equipment,” said Dvir Cohen, CEO of Memic. “This authorization is also just the beginning; it opens the door for our novel system to expand to additional indications that, until now, have been off-limits to robot-assisted surgery.”
Memic plans to pursue general surgery and transluminal indications for the Hominis platform and is developing artificial intelligence (AI)-enabled features to support all its surgical indications.
Such features are becoming more commonplace as endoscope tap the unimaginable capabilities of machine learning to improve disease diagnosis and treatment. Last fall, Olympus rolled out an artificial intelligence-powered platform in Europe designed to automatically spot suspicious lesions and polyps in real time during colonoscopies.
The ENDO-AID program works in combination with the EVIS X1 platform, launched in April 2020 in some parts of the world. The ENDO-AID’s machine learning algorithm alerts endoscopists when suspected colonic lesions (polyps, malignant neoplasms, adenomas) appear onscreen. (The ENDO-AID and EVIS XI products are not for sale in the United States at this time).
The company plans to use the AI program in the future in other regions for disease screenings in the esophagus, stomach, and other gastrointestinal organs.
“This has so much potential. I’m excited about the use of AI in endoscopy,” Doyle said. “It’s starting now with the detection and analysis of colon polyps. Machine learning technology is pretty well understood but to make it work well in endoscopy there needs to be good images annotated by good physicians, so they are teaching the computer what to look for. The clinical side of AI is very cool and I think that’s where a lot of the excitement will come in, but there are lots of other ways in which we can help enhance the endoscopy lab. I’m confident we’ll see more assistance from AI for helping diagnose disorders of the esophagus, colon, lungs, stomach, and other areas. No one expects AI to replace physicians. But AI can assist the physician or enhance his or her ability and bring everyone involved in care to a higher level of performance so they can demonstrably improve patient outcomes. And improving patient outcomes and safety is really the ultimate goal. That’s why we’re in this business.”
Sometimes, innovations are just that simple.
Consider the endoscope, for instance. Aside from a few rudimentary peeks, interior portions of the esophageal tract, stomach, and intestines were largely unexplored territories for several millennia, their function and foibles relegated mainly to imagination and conjecture.
Those realms would have remained uncharted, too, had it not been for a very basic request.
That appeal came from Tatsuro Uji, M.D., a physician at Tokyo University Hospital in Koishikawa. The year was 1949, and Uji—like many of his colleagues—was concerned about rising stomach cancer rates in Japan. Although tools had been developed over the previous 143 years to look inside the human body (including one that used a candle and angled mirrors), none could actually capture images.
Uji turned to Olympus Optical Co. Ltd. (now Olympus Corporation) for help in developing a camera that could photograph and examine the interior of a patient’s stomach. Mutsuo Sugiura, an Olympus engineer who was the same age as Uji, welcomed the request, reportedly saying, “As long as there is light, a lens, and a film, a camera can take pictures anywhere.”
Maybe so, but taking pictures inside the human body can be particularly challenging. Besides being safe for patients, internal body cameras also must comfortably navigate the gastrointestinal tract, quickly record snapshots, and produce sharp images for easier diagnosis. To achieve these objectives, the “gastrocamera” would require extremely small lenses, strong illumination sources, flexible materials (for tubes), and water leakage prevention capabilities.
“How to make an ultra-small camera that could be inserted into the stomach and still take clear photographs,” Toshio Nakatsubo, a former Olympus Corp. employee who contributed to the endoscope’s development, recalled in a 1998 Oyo-Buturi International interview. “That was the challenge. Being 1949, there was very little useful technology available, nor basic materials for construction for us [to] use.”
Despite all the hurdles, Olympus engineers developed a prototype in 1950 that featured a photographic lens located at the tip of a flexible tube, according to company history. Clinicians captured images on monochrome film by manually photoflashing a miniature light bulb in-vitro, and they wound the film by pulling a wire.
After various tweaks (one being a thinner tube), Olympus unveiled its gastrocamera—the GT-1—to great fanfare in late 1950. But the devices fared poorly in real clinical settings, often malfunctioning during photo sessions. Doctors soon inundated the company with product returns and repair requests.
While discouraging, the faulty products actually helped spawn advancements that eventually transformed the gastrocamera into an indispensable diagnostic tool. One such improvement was the incorporation of fiber optics (1964), which allowed doctors to examine the stomach in real time. Equally significant was the development of a video monitor system for simultaneously viewing those real-time images.
With subsequent advancements—transducers, HDTV, narrow-band imaging, self-navigation, autofluorescence, shape-locking overtubes, and light-scattering spectroscopy (among others)—the endoscope has proven its value to healthcare systems worldwide.
“If you go back to the dawn of endoscopy, it basically amounted to sticking a film camera at the end of a long tube in the stomach, snapping pictures blindly, and seeing what you get,” said Philip Doyle, executive director of Endoscopy Sales & Marketing for Olympus America Inc. “The technology has come a long way since then. After the original gastrocamera came fiber optic scopes, where you hold your eye to the top of the scope, you look through the fiber optic bundle, and you’re seeing in real-time what the scope is pointing at. From there, we went to video endoscopy, which was even better, and we progressed from standard to high-definition resolution. The resolution power of endoscopes has become better over time, we can see a lot more fine details and that’s helpful because some lesions may not be so obvious. Now you are better able to see what you otherwise could have overlooked with lesser image quality from the past. Narrow band imaging (NBI) helps to enhance the visibility of blood vessels, it filters white light into specific wavelengths that interact with hemoglobin and provides contrast for superficial blood vessels on the mucosal surface. It helps give physicians a better chance of detecting abnormal tissue and a better understanding of what they have found even before a pathologist gets back to them.”
Indeed, in the six decades since its birth, the endoscope has become a cornerstone of clinical treatment, begetting a multi-billion-dollar global market that is forecast to swell 8 percent annually over the next seven years, industry data indicate. Familiar market drivers are fueling the expansion: rising chronic disease caseloads (i.e., cancer, diabetes, heart conditions), as well as the increasing prevalence of (and preference for) minimally invasive (MI) surgery.
“There are a few major trends shaping the global endoscopic procedures market. The first is the broadly increasing adoption rate of minimally invasive or endoscopic procedures within the surgical setting,” said Charles Golub, market development manager at Saint-Gobain Life Sciences Medical Components, a Northborough, Mass.-based contract manufacturer focused on customized material solutions from silicone, thermoplastic elastomers, and polyvinyl chloride. “Previously, standard treatment options involved highly invasive procedures; recently though, endoscopic procedures are becoming more routine, which is enabling quicker patient recovery times and reducing the burden on hospitals.”
It’s also enabling new product innovations. Last fall, Olympus introduced two U.S. Food and Drug Administration (FDA)-cleared colonoscopes that expand the company’s capabilities in MI procedures, including endoscopic submucosal dissection (ESD).
The PCF-H190T endoscope is a slimmer, short-bending, high-definition device made for turning tight corners. Designed to help classify colorectal polyps, the PCF-H190T features better retroflexion capability than its predecessors (the technique is commonly used to detect distal rectal polyps), and a channel layout appropriate for passing through therapeutic accessories, such as for hemostasis or polypectomy. The device also is inserted more easily into the rectum, for better patient comfort.
Both the PCF-H190T and its sister product, the PCF-HQ190, are used with the EVIS EXERA III imaging platform. The PCF-HQ190 contains dual-focus hardware for visualizing the intestine’s mucus membranes in a 170-degree field-of-view. The scope’s NBI and its near focus mode have a greater than 90 percent agreement with pathological analysis in assigning post-polypectomy surveillance after a colonoscopy, according to Olympus.
The PCF-HQ190 also features Responsive Insertion Technology to facilitate complete colonoscopies and reduced cecal intubation times through better maneuverability. Its built-in ScopeGuide Function provides real-time 3D visualization of the device’s position and configuration during insertion, including identifying scope loops as they form.
“There are many minimally invasive interventions that can be done via endoscopy. These procedures tend to involve less trauma to the patient, decreased recovery time, and lower costs to the healthcare system,” Doyle noted. “Endoscopic ultrasound is one such intervention that comes to mind. It’s a procedure guided by ultrasound, using a special endoscope with an ultrasound probe attached. There’s also endoscopic submucosal dissection, or ESD, and peroral endoscopic myotomy. These kinds of procedures would have required surgical intervention in the past.”
Endobronchial ultrasound would have as well. Sampling mediastinal lymph nodes or peribronchial tumors used to require an invasive chest incision, but endobronchial ultrasound (EBUS) procedures avoid any cuts, relying instead on real-time imaging to access smaller lymph nodes for biopsy.
Two types of EBUS techniques are used for diagnosing lung cancer: radial and transbronchial needle aspiration. Radial EBUS typically produces more accurate nodule samples in accessibly difficult areas while EBUS-TBNA helps clinicians “see” beyond the lungs’ walls.
A Radial EBUS procedure is performed by inserting a miniature ultrasound probe through the working channel of a flexible bronchoscope or catheter (guide sheath), according to Olympus. Real-time imaging of the surrounding tissue enables doctors to determine a lesion’s precise location and size. Upon identification, the probe is withdrawn and a biopsy sampling device is inserted down the scope to collect tissue samples for biopsy.
EBUS-TBNA requires more professional training and is a longer procedure, but usually yields larger samples than conventional TBNA. This approach uses an ultrasound transducer integrated into a flexible fiberoptic bronchoscope for viewing images. Once a satisfactory image is obtained, a biopsy needle with a pre-set stylet length is passed through the bronchoscope’s proximal port, then under direct and real-time vision, is inserted through the endobronchial mucosa and into the lymph node or mass. Three needle aspirations per lymph node is recommended for non-small cell lung cancer screenings.
EBUS-TBNA procedures have correctly staged and diagnosed lung cancer more than 95 percent of the time, and multiple prospective studies have shown a similarly high accuracy rate for detecting extra-thoracic malignancies and lymphomas (85-95 percent and 91-97 percent, respectively).
Olympus and PENTAX Medical are working to boost those scores and improve the EBUS-TBNA technique with newly-launched products designed to enhance endobronchial ultrasound access, visualization, and bronchoscope control.
Olympus’ BF-UC190F next-generation EBUS bronchoscope, for example, offers improved lymph node access in hard-to-reach areas and a compact distal tip (6.6mm diameter) for smoother insertion and easier maneuvering in the lung. Launched in May, the device features an increased scope angulation (from 120 degrees to 160 degrees), allowing better positioning of the scope tip relative to the targeted tissue.
Additionally, a more perpendicular needle orientation allows for smoother target penetration between cartilage rings and other critical anatomy, the company claims. The BF-UC190F also generates higher-resolution images, and possesses a decreased forward oblique angle (20 degrees) that keeps the endoscopic image in full field view during airway navigation.
PENTAX Medical’s newest EBUS-TBNA bronchoscope enhances imaging as well. The company claims its EB19-J10U Ultrasound Video Bronchoscope, released last September, provides “crystal clear visualization” of the bronchial anatomy and improved procedural efficiency for tissue sampling.
According to PENTAX, the EB19-J10U is ergonomically designed with a focus on patient comfort, minimum stress on the operator’s hand, and improved endoscopic visibility. Its small, easily maneuverable ultrasound transducer at the bronchoscope’s tip is permanently visible during the procedure, allowing clinicians to confirm its airway positioning. Lung and lymph node imaging is improved with a wide 75-degree field of vision, and a new 2.2-mm working channel is compatible with 19-Gauge needles, a relatively recent development.
“EBUS is recognized as the gold standard for minimally invasive tissue acquisition in pulmonology,” PENTAX Medical Americas President and CEO David Woods said upon the EB19-J10U’s market release. “Combined with Hitachi Ultrasound Scanners, the EB19-J10U reaffirms our commitment to diagnostic and interventional Pulmonology. PENTAX is globally focused on innovations that not only help improve clinical outcomes and reduce the cost of care delivery, but also enhance our customers’ satisfaction. We think the EB19-J10U is a significant building block for this strategy.”
Auris Health Inc. is employing the same strategy, but with a different building block. The company’s Monarch Platform harnesses the proficiencies of robotic technology to better examine the lungs. The platform combines traditional endoscopic views of the body’s airways with computer-assisted navigation based on 3D anatomical models to provide continuous vision during diagnostic or therapeutic bronchoscopy procedures.
The U.S. Food and Drug Administration (FDA)-cleared Monarch system features a video game-style controller that is used to navigate a flexible robotic endoscope to the periphery of the lung with improved reach, vision, and control. The system is designed to more accurately access, diagnose, and treat small, hard-to-reach peripheral lung nodules.
“Conventional tools are limited in their ability to reach and get an early and accurate diagnosis in lung modules,” noted Eric Davidson, president of Flexible Robotics at Auris Health Inc., a company Ethicon Inc., part of the Johnson & Johnson Medical Device Companies, acquired in February 2019 for $3.4 billion. “With Monarch, we are trying to play a role in extending a physician’s capabilities and better define what is possible endoscopically. And we’re starting that journey with lung cancer—specifically, providing bronchoscopic visualization of and access to patient airways for diagnostic and therapeutic procedures. Monarch is designed to overcome the limitations of current diagnostic capabilities by providing visualization and access for the biopsy needle to go where the physician wants it. Lung cancer is a disease that, if caught late, has quite poor survival rates.”
Auris is hopeful the Monarch Platform will eventually boost those rates. An inaugural study of the technology shows promise, with results indicating the system reached target lung lesions in more than 96 percent of patients (52 of 54 people across five sites). Pneumothorax (collapsed lung) occurred in two patients, one of which required the placement of a tube to drain air or fluid from the chest.
The study’s overall diagnostic yield was 74.1 percent, and a 70 percent yield was achieved in lung nodules located outside the patient’s airway. Non-robotic technology reported 30-40 percent diagnostic yields.
“Lung cancer kills more people worldwide than the next three cancers combined. There are 1.5 million lung nodules that present each year in the United States, and they can be deep inside the lung,” Davidson told Medical Product Outsourcing. “That is a very complex area in which to diagnose and treat. With these hard-to-reach nodules, conventional tools make it hard to diagnose lung cancer. For years, there’s been an effort in healthcare to diagnose the disease earlier and more accurately. Monarch is an enabling endoscopic technology—it’s a means to access lung nodules safely and accurately for either diagnostic or therapeutic intent.”
Certainly, the Monarch Platform provides a means to a very specific end, but it’s not the only “enabling” technology of its kind. Natural orifice transluminal endoscopic surgery (NOTES) is becoming a more preferential MI surgical option, as it capitalizes on the body’s natural orifices (mouth, anus, vagina, urethra) to access the abdominal cavity.
NOTES is performed using a flexible endoscope that is advanced into the peritoneal cavity after puncturing an internal organ such as the stomach, colon, vagina, or bladder. Conventional endoscopic instruments are then introduced through the flexible device’s working channels to conduct the procedure.
While NOTES provides all the advantages of laparoscopic surgery, it also offers several added benefits, including lower anesthesia requirements, less pain and scarring, faster recovery, and a fewer incidents of wound-related complications.
“Many endoscopic surgeries started using robotic-assisted devices years ago, specifically some of the thoracic procedures for lung cancer,” Saint Gobain’s Golub stated. “Newer procedures are rapidly adopting this technology, such as the natural orifice (NOTES) procedures. As these devices gain more acceptance and popularity with physicians and practitioners, I anticipate we’ll see many new challenges arise in the design and ease of use for these endoscopic devices.”
Some of those challenges have already surfaced: hysteresis reduction, enhanced maneuverability and degrees of freedom, internal instrument exchange, broader operative capabilities, and part miniaturization.
One of the big challenges with miniaturization is heat dissipation for the LED lighting on the tip of many endoscopes,” said Arthur Roti, general manager of Instrument Technology Inc., a Westfield, Mass.-headquartered designer, developer, and manufacturer of borescopes, fiberscopes, and videoscopes.
Researchers are working fervently to overcome such barriers as interest in gastric applications for NOTES builds among surgeons and advanced endoscopists.
EndoMaster Pte. Ltd., for example, is working to develop an endoscopic robotic system that removes cancerous gastric and intestinal tumors without incisions. The Singapore firm’s flexible endoscopic robot—EndoMaster EASE (Endoluminal Access Surgical Efficacy) System—consists of a flexible platform and endoscopic imaging system that allows the passage of two minute robotic arms to achieve tissue retraction and dissection in ESD (endoscopic submucosal dissection) procedures, according to the Chinese University of Hong Kong (CU Medicine).
The university used the EndoMaster EASE last spring to conduct the world’s first clinical trial on robotic colorectal ESD, an advanced, less invasive endoscopic treatment for early gastrointestinal cancer. Six patients underwent colorectal ESD with the EASE System and no instances of perforation were recorded. Patients resumed a normal diet after one day and were discharged after two days, CU Medicine reported.
“From our initial observation, the flexible endoscopic robotic system is a very promising armamentarium for performing ESD and treating early-stage colorectal cancer,” said Professor Philip Wai Yan Chiu, director of the university’s Jockey Club Minimally Invasive Surgical Skills Center. “Our clinical trial is still currently underway and we will continue to enroll suitable patients to participate.”
The EndoMaster EASE System is neither CE Marked nor FDA approved.
The Hominis platform is off-limits in Europe, too, but is blessed by the FDA for natural orifice laparoscopic-assisted transvaginal benign surgical procedures, including benign hysterectomy. Developed by Tel Aviv, Israel-based Memic Innovative Surgery Ltd., Hominis is designed to remove the uterus using minimally invasive surgical instruments inserted through the vagina, as well as a video camera inserted laparoscopically through a small incision on the abdomen.
The Hominis (Latin for humanoid) system is touted as the “first and only” FDA-authorized surgical robotic platform featuring miniature humanoid-shaped robotic arms that provide human-level dexterity, multi-planar flexibility, and 360 degrees of articulation. The system’s biomimetic instruments aim to replicate the motions and capabilities of a surgeon’s arms, with shoulder, elbow, and wrist joints. Multiple instruments can be introduced to the body through a single portal and the 360-degree articulation offers obstacle avoidance as well as optimal access and working angles.
“We are pleased to receive FDA De Novo authorization of our Hominis system, which offers a small, cost-effective and less invasive option over current robotic instruments limited to straight shaft and single wrist designs and controlled with large, complex and expensive equipment,” said Dvir Cohen, CEO of Memic. “This authorization is also just the beginning; it opens the door for our novel system to expand to additional indications that, until now, have been off-limits to robot-assisted surgery.”
Memic plans to pursue general surgery and transluminal indications for the Hominis platform and is developing artificial intelligence (AI)-enabled features to support all its surgical indications.
Such features are becoming more commonplace as endoscope tap the unimaginable capabilities of machine learning to improve disease diagnosis and treatment. Last fall, Olympus rolled out an artificial intelligence-powered platform in Europe designed to automatically spot suspicious lesions and polyps in real time during colonoscopies.
The ENDO-AID program works in combination with the EVIS X1 platform, launched in April 2020 in some parts of the world. The ENDO-AID’s machine learning algorithm alerts endoscopists when suspected colonic lesions (polyps, malignant neoplasms, adenomas) appear onscreen. (The ENDO-AID and EVIS XI products are not for sale in the United States at this time).
The company plans to use the AI program in the future in other regions for disease screenings in the esophagus, stomach, and other gastrointestinal organs.
“This has so much potential. I’m excited about the use of AI in endoscopy,” Doyle said. “It’s starting now with the detection and analysis of colon polyps. Machine learning technology is pretty well understood but to make it work well in endoscopy there needs to be good images annotated by good physicians, so they are teaching the computer what to look for. The clinical side of AI is very cool and I think that’s where a lot of the excitement will come in, but there are lots of other ways in which we can help enhance the endoscopy lab. I’m confident we’ll see more assistance from AI for helping diagnose disorders of the esophagus, colon, lungs, stomach, and other areas. No one expects AI to replace physicians. But AI can assist the physician or enhance his or her ability and bring everyone involved in care to a higher level of performance so they can demonstrably improve patient outcomes. And improving patient outcomes and safety is really the ultimate goal. That’s why we’re in this business.”