Priyank Kishor, Global Product Manager—PulseStaking, Branson at Emerson11.04.20
With each new generation of medical device technology, medical professionals and patients gain the ability to not only monitor and manage improved health and wellness, but also to collect data, identify possible problems, and deliver targeted treatments more rapidly and precisely. For medical device manufacturers, the challenge of new product development cuts two ways. On one hand, there’s the challenge of designing devices that provide greater capability, operate with greater precision, and communicate more precise and descriptive data more rapidly (when needed). On the other hand, there’s the challenge of manufacturing and assembling these ever more capable and component-dense devices into structures often comprised of plastic parts.
To arrange and secure so many small and often sensitive electronic items into component-dense plastic structures, device manufacturers are pushing the boundaries of two widely used plastic assembly technologies: staking and swaging. Staking involves placing components onto small posts in a plastic part, then reforming or “staking” the exposed posts using heat and force into flattened, rivet-like disks that lock the components in place. The other process, swaging, typically involves using heat and force to bend the flap or rim of a plastic part laterally around the edges of a component to secure it in place.
Both of these technologies are widely used in the assembly of medical devices because they enable plastic structures to capture and secure components made from many materials: plastics, metals, fabrics and filter media, as well as printed circuit boards (PCBs), switches, and electronics. Thermoplastic stakes or swages can be made quickly and at relatively low cost, without the need for labor-intensive mechanical fastening or expensive adhesive fastening processes. But as device designs have evolved and more and more delicate components are assembled into the newest devices, device manufacturers find the two traditional swaging and staking technologies—thermal staking and ultrasonic staking—face technical limitations.
A newer approach, called PulseStaking, addresses many of these limitations and is now becoming available worldwide. The technology was developed by HTE Engineering Services Ltd., which was acquired in October 2018 by Emerson. This technology not only performs all of the same staking and swaging tasks as current heated-tip and ultrasonic processes, but is also readily applicable to even the most complex, component-dense designs. PulseStaking handles multiple, closely spaced features on geometrically complex parts. It reliably assembles heat- and vibration-sensitive components. And, it works with a wider range of plastic materials.
Three Types of Staking Technology
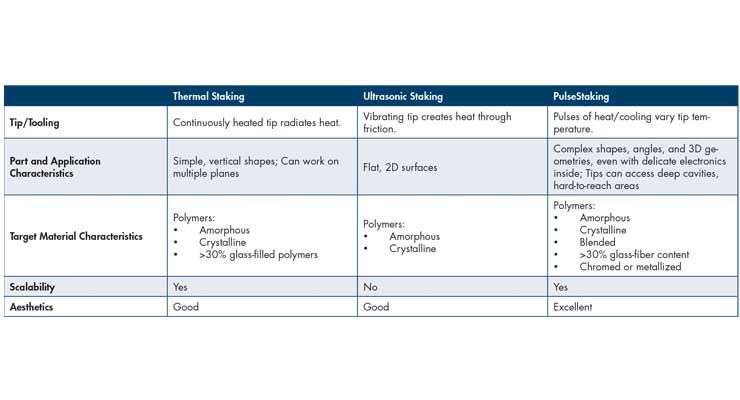
Thermal staking and swaging use a continuously heated tip. When pressed into plastic, the tip melts the plastic, forming it according to the shape of the tip. When the tip is removed, the part is left to cool. However, since this process relies on one tip and one temperature, it can be difficult to balance the speed of the heat input needed for good melting and forming with the desirability of rapid cooling to preserve the strength and aesthetics of the finished shape. Target applications include simple, vertical shapes that allow direct vertical access. Since thermal tips constantly radiate heat, plastic part posts and bosses must be adequately spaced to prevent unintended damage. Similarly, the placement of PCBs, sensors, or electronics must incorporate clearances sufficient to prevent them from heat-related damage when they are attached using swages or stakes.
Ultrasonic swaging or staking employs vibratory energy—also applied through metal tooling—to create frictional heat used to melt and form plastic stakes or swages. Similar to thermal staking, target applications for ultrasonic staking include plastic parts with simple shapes and flat, 2D surfaces. Unlike thermal staking or swaging, the ultrasonics work very well with heat-sensitive fabrics and components because melt heat is highly localized and carefully controlled. However, the vibratory nature of the process poses a damage risk to electronic components.
The PulseStaking process opens up new application possibilities because it uses and applies heat much more selectively. Unlike thermal staking tools, which continuously radiate high heat in all directions before, during, and after a stake, PulseStaker tips heat and cool instantly—and only when needed—to apply heat in a highly controlled and localized manner. Each tip combines an electrical heating element with a compressed-air cooling system. In operation, tips go through a cycle of multiple heating, cooling, and pause or “dwell” intervals to prevent overheating and precisely manage tip and plastic temperature until each stake is completed.
The PulseStaking Cycle
PulseStaking cycles can be managed using either open- or closed-loop controls. Cycles typically consist of eight discrete parts—three intervals of heating, three dwell intervals, and two intervals of cooling. Cycles may last anywhere from several seconds to 15 seconds or longer.
Heating: When the PulseStaking tip touches the plastic point to be staked, pulses of electrical current are passed through the tip, which rapidly heats up (1st heating) and begins to soften the plastic. Then, after a brief nonpowered dwell period that allows tip temperature to equalize, the tip reheats (2nd heating) and is pushed onto the plastic, where it displaces the melted plastic and forms the swage or stake using the contours of the PulseStaking tip.
Dwell or Weld: For brief periods, the current that heats the tip is switched off. This enables the rapid build-up of heat from the tip sufficient time to be conducted into the surrounding plastic. These periods promote more even heating of the target area and prevent overheating or burning of the forming plastic.
Cooling: To cool the melted, forming plastic and enable it to set quickly, a compressed-air blast is directed to the plastic from the inside center of the tip.
Reheat: When the tip and plastic cool after forming, the plastic can sometimes stick, preventing the tip from being pulled away cleanly. To overcome this problem, a reheat interval is used. Current is briefly pulsed through the tip to heat it just prior to extraction, softening the surrounding plastic and enabling a clean tip extraction and an excellent cosmetic result in the finished bond.
Process Benefits
The unique characteristics of PulseStaking technology enable it to perform all of the same types of stakes or swages as previous technology, but with a higher degree of aesthetic consistency and quality. However, the greatest strength of the technology for medical device makers lies in its ability to tackle applications where other staking and swaging technology falls short, including:
PulseStaking technology offers advantages for working with complex, contoured, and closely aligned part features because of the unique tip design, which allows instant and localized heating and cooling. When applications require multi-tip tooling, pulsing tips can be positioned much more closely and in more complex configurations than traditional heated tips. Further, since pulsing tips heat up only during their short operating cycle, there’s no risk of unintended radiant heating, even if tooling or tips pass very close to nontarget surfaces or heat-sensitive components.
The unique ability to vary tip and plastic temperatures within a staking or swaging cycle enables the PulseStaker process to deliver both clean (particulate-free) and aesthetically superior results for a wide range of materials, even those with levels of glass fill exceeding 30 percent. Glass fibers tend to stick to traditional thermal tips and pull away from finished stake when thermal tips are removed. However, since the PulseStaking process allows for temperature changes within a cycle, its tip can briefly reheat to release cleanly before final cooling takes place.
Compared to other forming technologies, PulseStaking technology can also join a wider variety of different materials to plastic components or housings, including metal shims; plastic keys or buttons; filters, fibrous cloth, or insulating materials; PCBs, electronics, or sensors; and fragile glass or ceramic elements.
Beyond the realm of current applications, PulseStaking technology opens up a variety of new part design and production options. First, heating tips are available in many standard and custom shapes (e.g., domed, rectangular, lozenge) and can be operated singly or, if production and cycle times require it, densely grouped into larger tools that can perform multiple forming operations at once. Second, the localized heating characteristics of each tip enable forming operations on complex or angled surfaces, and can even reach into deep cavities or other difficult-to-access areas without risk of damage to nontarget surfaces.
Priyank Kishor is global product manager, PulseStaking, for Branson at Emerson. He leads global marketing and product strategy for the PulseStaker platform and other non-ultrasonic products in the Branson assembly technology portfolio. He has a decade of experience in managing programs and teams involved in global product development and international marketing. Kishor holds an MBA in marketing from the University of Mannheim, Germany, and a bachelor’s degree in engineering from Birla Institute of Technology in Mesra, India.
To arrange and secure so many small and often sensitive electronic items into component-dense plastic structures, device manufacturers are pushing the boundaries of two widely used plastic assembly technologies: staking and swaging. Staking involves placing components onto small posts in a plastic part, then reforming or “staking” the exposed posts using heat and force into flattened, rivet-like disks that lock the components in place. The other process, swaging, typically involves using heat and force to bend the flap or rim of a plastic part laterally around the edges of a component to secure it in place.
Both of these technologies are widely used in the assembly of medical devices because they enable plastic structures to capture and secure components made from many materials: plastics, metals, fabrics and filter media, as well as printed circuit boards (PCBs), switches, and electronics. Thermoplastic stakes or swages can be made quickly and at relatively low cost, without the need for labor-intensive mechanical fastening or expensive adhesive fastening processes. But as device designs have evolved and more and more delicate components are assembled into the newest devices, device manufacturers find the two traditional swaging and staking technologies—thermal staking and ultrasonic staking—face technical limitations.
A newer approach, called PulseStaking, addresses many of these limitations and is now becoming available worldwide. The technology was developed by HTE Engineering Services Ltd., which was acquired in October 2018 by Emerson. This technology not only performs all of the same staking and swaging tasks as current heated-tip and ultrasonic processes, but is also readily applicable to even the most complex, component-dense designs. PulseStaking handles multiple, closely spaced features on geometrically complex parts. It reliably assembles heat- and vibration-sensitive components. And, it works with a wider range of plastic materials.
Three Types of Staking Technology
Thermal staking and swaging use a continuously heated tip. When pressed into plastic, the tip melts the plastic, forming it according to the shape of the tip. When the tip is removed, the part is left to cool. However, since this process relies on one tip and one temperature, it can be difficult to balance the speed of the heat input needed for good melting and forming with the desirability of rapid cooling to preserve the strength and aesthetics of the finished shape. Target applications include simple, vertical shapes that allow direct vertical access. Since thermal tips constantly radiate heat, plastic part posts and bosses must be adequately spaced to prevent unintended damage. Similarly, the placement of PCBs, sensors, or electronics must incorporate clearances sufficient to prevent them from heat-related damage when they are attached using swages or stakes.
Ultrasonic swaging or staking employs vibratory energy—also applied through metal tooling—to create frictional heat used to melt and form plastic stakes or swages. Similar to thermal staking, target applications for ultrasonic staking include plastic parts with simple shapes and flat, 2D surfaces. Unlike thermal staking or swaging, the ultrasonics work very well with heat-sensitive fabrics and components because melt heat is highly localized and carefully controlled. However, the vibratory nature of the process poses a damage risk to electronic components.
The PulseStaking process opens up new application possibilities because it uses and applies heat much more selectively. Unlike thermal staking tools, which continuously radiate high heat in all directions before, during, and after a stake, PulseStaker tips heat and cool instantly—and only when needed—to apply heat in a highly controlled and localized manner. Each tip combines an electrical heating element with a compressed-air cooling system. In operation, tips go through a cycle of multiple heating, cooling, and pause or “dwell” intervals to prevent overheating and precisely manage tip and plastic temperature until each stake is completed.
The PulseStaking Cycle
PulseStaking cycles can be managed using either open- or closed-loop controls. Cycles typically consist of eight discrete parts—three intervals of heating, three dwell intervals, and two intervals of cooling. Cycles may last anywhere from several seconds to 15 seconds or longer.
Heating: When the PulseStaking tip touches the plastic point to be staked, pulses of electrical current are passed through the tip, which rapidly heats up (1st heating) and begins to soften the plastic. Then, after a brief nonpowered dwell period that allows tip temperature to equalize, the tip reheats (2nd heating) and is pushed onto the plastic, where it displaces the melted plastic and forms the swage or stake using the contours of the PulseStaking tip.
Dwell or Weld: For brief periods, the current that heats the tip is switched off. This enables the rapid build-up of heat from the tip sufficient time to be conducted into the surrounding plastic. These periods promote more even heating of the target area and prevent overheating or burning of the forming plastic.
Cooling: To cool the melted, forming plastic and enable it to set quickly, a compressed-air blast is directed to the plastic from the inside center of the tip.
Reheat: When the tip and plastic cool after forming, the plastic can sometimes stick, preventing the tip from being pulled away cleanly. To overcome this problem, a reheat interval is used. Current is briefly pulsed through the tip to heat it just prior to extraction, softening the surrounding plastic and enabling a clean tip extraction and an excellent cosmetic result in the finished bond.
Process Benefits
The unique characteristics of PulseStaking technology enable it to perform all of the same types of stakes or swages as previous technology, but with a higher degree of aesthetic consistency and quality. However, the greatest strength of the technology for medical device makers lies in its ability to tackle applications where other staking and swaging technology falls short, including:
- Complex device designs with plastic parts that require swaging or staking on varied surface contours or that require multiple swages or stakes in close proximity to other heat-sensitive features.
- Devices that require swaging or staking of parts made with any of a growing number of advanced, blended, glass-reinforced, or chromed/metallized plastics.
- Devices with plastic parts that must capture and support delicate, heat-sensitive materials or assemblies made from plastics, films or fabrics, ceramics, or metals.
- Parts that must capture and hold small or closely spaced, heat- or vibration-sensitive electronic components, such as PCBs, soldered components, or sensors.
PulseStaking technology offers advantages for working with complex, contoured, and closely aligned part features because of the unique tip design, which allows instant and localized heating and cooling. When applications require multi-tip tooling, pulsing tips can be positioned much more closely and in more complex configurations than traditional heated tips. Further, since pulsing tips heat up only during their short operating cycle, there’s no risk of unintended radiant heating, even if tooling or tips pass very close to nontarget surfaces or heat-sensitive components.
The unique ability to vary tip and plastic temperatures within a staking or swaging cycle enables the PulseStaker process to deliver both clean (particulate-free) and aesthetically superior results for a wide range of materials, even those with levels of glass fill exceeding 30 percent. Glass fibers tend to stick to traditional thermal tips and pull away from finished stake when thermal tips are removed. However, since the PulseStaking process allows for temperature changes within a cycle, its tip can briefly reheat to release cleanly before final cooling takes place.
Compared to other forming technologies, PulseStaking technology can also join a wider variety of different materials to plastic components or housings, including metal shims; plastic keys or buttons; filters, fibrous cloth, or insulating materials; PCBs, electronics, or sensors; and fragile glass or ceramic elements.
Beyond the realm of current applications, PulseStaking technology opens up a variety of new part design and production options. First, heating tips are available in many standard and custom shapes (e.g., domed, rectangular, lozenge) and can be operated singly or, if production and cycle times require it, densely grouped into larger tools that can perform multiple forming operations at once. Second, the localized heating characteristics of each tip enable forming operations on complex or angled surfaces, and can even reach into deep cavities or other difficult-to-access areas without risk of damage to nontarget surfaces.
Priyank Kishor is global product manager, PulseStaking, for Branson at Emerson. He leads global marketing and product strategy for the PulseStaker platform and other non-ultrasonic products in the Branson assembly technology portfolio. He has a decade of experience in managing programs and teams involved in global product development and international marketing. Kishor holds an MBA in marketing from the University of Mannheim, Germany, and a bachelor’s degree in engineering from Birla Institute of Technology in Mesra, India.