Maria Shepherd, President and Founder, Medi-Vantage10.01.20
Recent publications suggest the U.S. performs more robotic surgery than every other country in the world. Using robotic device industry financial statements, robotic procedure volumes exceeded 600,000 in 2017, with the largest share of the market attributable to general surgery.1 These publications indicate the footprint in U.S. hospitals for surgical robotics will continue growing at this pace for the next decade.
Robotic surgery continues to broaden use across most surgical procedures. Arguments are made that it is more expensive2 and possibly less efficacious3 than other traditional surgical procedures, such as laparoscopic and open surgery. The FDA has issued a warning against the use of robotic surgery for breast and cervical cancers.4 In this warning, FDA expressed concerns about the lack of broad clinical data for the use of robotic surgery in these procedures. In a population-based dataset from the state of Michigan between Jan. 1, 2012, through June 30, 2018, surgical trends in the use of robotic surgery for common procedures where traditional laparoscopic surgery was considered the gold standard was tracked after hospitals launched a robotic surgery program. Data were examined from March 1, 2019 to April 19, 2019.
Why This Is Important
Everyone wants a piece of the robotics surgery market. For example, in 2019, Bob White, EVP and president of Medtronic’s minimally invasive therapies group stated, “This isn’t just about launching a robot, this is about building a robotics business.” He added, “When Porsche decided to enter the electric car market, they [brought] with them tremendous experience in automotive innovation. They had never built an electric car, but they understood automotive…[Medtronic understands] surgery better than any other company in the world and that’s what we bring to the table.”5
In addition, White indicated Medtronic had designed a surgical robot to address the cost and utilization barriers that are current obstacles to robotic market growth. He mentioned a question he often gets, “Well Bob, how do you feel about competing in this 2 percent space?” His response to it was, “This is about 98 percent of the procedures that aren’t being done. That’s about increasing market access and that’s something we do very well.”
This philosophy toward market adoption matters. In the JAMA Michigan study (n=169,404), the use of robotic surgery increased from 1.8 percent of all procedures in 2012 to 15.1 percent in 2018 (Table 1).1 Some procedures, like inguinal hernia repair, saw an increase in the use of robotic surgery from 0.7 percent in 2012 to 28.8 percent in 2018.
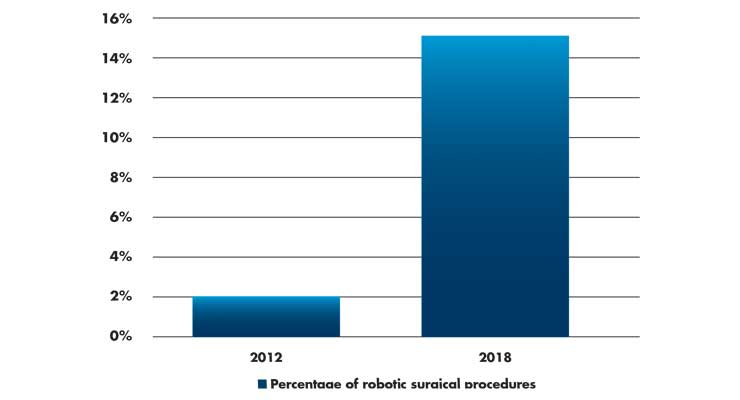
Table 1: Percentage of procedures that used robotic surgical technology as compared to all surgical procedures. Data derived from a cohort study of 169,404 patients in 73 Michigan hospitals.1
The 20-Year History of Surgical Robotics
In 2000, Intuitive Surgical launched the first robotic surgical platform in the U.S. It was cleared by the FDA for general laparoscopic surgery.6 Since then, more than 5,000 robotic surgery systems have been installed in hospitals around the world and approximately 39,000 surgeons have been trained on robotic surgery systems. Only 2 percent of procedures globally are being performed using surgical robots, but estimates for U.S. use place the total number of procedures at approximately 10 percent. This means of the more than 5,000 installed units, surgical robots are being used less than once a day on average around the world.7
The global surgical robotics market is forecast to grow from $3.9 billion in 2018 to $6.5 billion by 2023 (Table 2), growing at a CAGR of 10.4 percent, estimates one market research company.8 Top vendors are led by Intuitive Surgical and also include Stryker and Mazor Robotics (now a part of Medtronic). Intuitive Surgical’s revenues were reported at $2.1 billion in the first six months of 2019, with 18 percent growth YOY. The da Vinci robot is now a fourth-generation system and priced at $1 million to $2 million per system. Intuitive had an installed base of 5,270 machines globally as of June 30, 2019, and 689 da Vinci robots were sold within Asia, with more than 100 in India.7
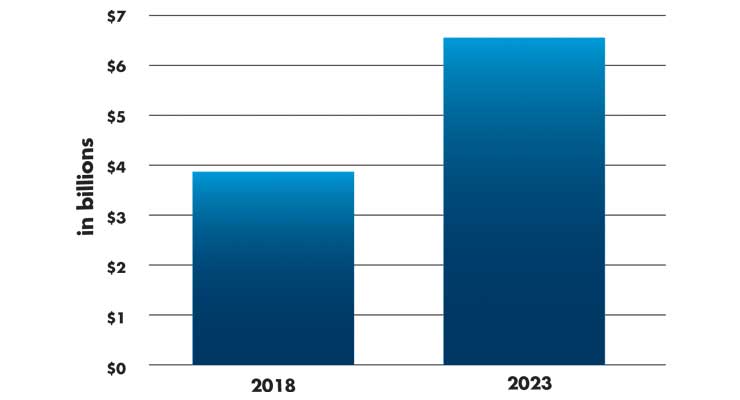
Table 2: Projected global growth of the surgical robotics market8
A report from the Royal College of Surgeons notes the changes in the beginning and ensuing adoption of robotic surgery over the past 20 years, which was created and dominated by the da Vinci surgical robotics platform.9 The paper describes the current commercially available systems and new, innovative systems in development. With a nod to the expense of robotics systems, the authors indicate a higher level of competition should result in reduced costs and improved market access. Less likely, the authors suggest, is collaboration between surgical robotic system manufacturers to standardize basic features, which would be desirable to surgeons, but would reduce differentiation and switching costs between systems. Several novel surgical robotics systems are in development for applications in gynecological, colorectal, cardiothoracic, upper GI, renal, urological, and general surgery.
Where’s the Growth Coming From?
As the use of surgical robotics increased, the Michigan study procedural trend saw a decline in laparoscopic surgery from 53.2 percent prior to adopting robotic surgery to 51.3 percent after the surgical robots were installed. In addition, growth drivers include: rising technological advancement in surgical robotic tools in developed countries, an increase in the number of procedures in North America and Asia Pacific, expanding healthcare spending in emerging countries, and a positive regulatory scenario and the aging of the baby boomer generation in the U.S.10
Don’t Forget the Adverse Events
Even with broad adoption of surgical robotic systems, adverse events (death, patient injuries, and device malfunctions) are still seen during procedures. Most are preventable incidents and need to be addressed in the design of surgical robotic platforms. During the study period, (2000-2013), 10,624 adverse events were reported (Table 3). Surprisingly, the number of injury and deaths per procedure have remained relatively constant over this period.11
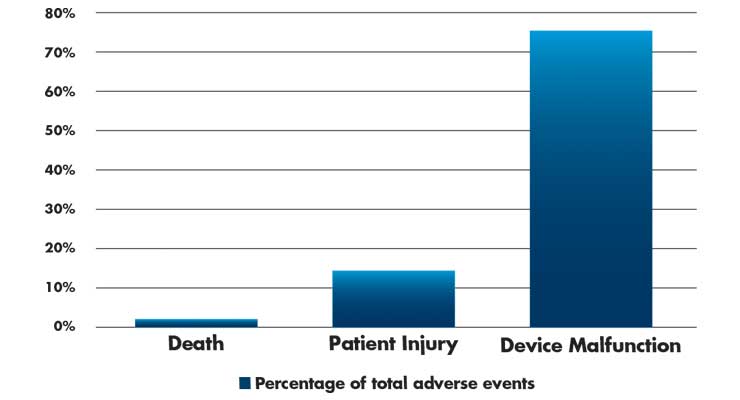
Table 3: Adverse events (n=10,624) in robotic surgery from 2000-2013.11
The Medi-Vantage Perspective
Robots are here to stay, and robotic surgery will continue to develop as a critical surgical skill. The question is, which medtech company will have the ability to address the entire market, instead of smaller segments?
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses, speaks regularly at medtech conferences, and can be reached at mshepherd@medi-vantage. Visit her website at www.medi-vantage.com.
Robotic surgery continues to broaden use across most surgical procedures. Arguments are made that it is more expensive2 and possibly less efficacious3 than other traditional surgical procedures, such as laparoscopic and open surgery. The FDA has issued a warning against the use of robotic surgery for breast and cervical cancers.4 In this warning, FDA expressed concerns about the lack of broad clinical data for the use of robotic surgery in these procedures. In a population-based dataset from the state of Michigan between Jan. 1, 2012, through June 30, 2018, surgical trends in the use of robotic surgery for common procedures where traditional laparoscopic surgery was considered the gold standard was tracked after hospitals launched a robotic surgery program. Data were examined from March 1, 2019 to April 19, 2019.
Why This Is Important
Everyone wants a piece of the robotics surgery market. For example, in 2019, Bob White, EVP and president of Medtronic’s minimally invasive therapies group stated, “This isn’t just about launching a robot, this is about building a robotics business.” He added, “When Porsche decided to enter the electric car market, they [brought] with them tremendous experience in automotive innovation. They had never built an electric car, but they understood automotive…[Medtronic understands] surgery better than any other company in the world and that’s what we bring to the table.”5
In addition, White indicated Medtronic had designed a surgical robot to address the cost and utilization barriers that are current obstacles to robotic market growth. He mentioned a question he often gets, “Well Bob, how do you feel about competing in this 2 percent space?” His response to it was, “This is about 98 percent of the procedures that aren’t being done. That’s about increasing market access and that’s something we do very well.”
This philosophy toward market adoption matters. In the JAMA Michigan study (n=169,404), the use of robotic surgery increased from 1.8 percent of all procedures in 2012 to 15.1 percent in 2018 (Table 1).1 Some procedures, like inguinal hernia repair, saw an increase in the use of robotic surgery from 0.7 percent in 2012 to 28.8 percent in 2018.

Table 1: Percentage of procedures that used robotic surgical technology as compared to all surgical procedures. Data derived from a cohort study of 169,404 patients in 73 Michigan hospitals.1
The 20-Year History of Surgical Robotics
In 2000, Intuitive Surgical launched the first robotic surgical platform in the U.S. It was cleared by the FDA for general laparoscopic surgery.6 Since then, more than 5,000 robotic surgery systems have been installed in hospitals around the world and approximately 39,000 surgeons have been trained on robotic surgery systems. Only 2 percent of procedures globally are being performed using surgical robots, but estimates for U.S. use place the total number of procedures at approximately 10 percent. This means of the more than 5,000 installed units, surgical robots are being used less than once a day on average around the world.7
The global surgical robotics market is forecast to grow from $3.9 billion in 2018 to $6.5 billion by 2023 (Table 2), growing at a CAGR of 10.4 percent, estimates one market research company.8 Top vendors are led by Intuitive Surgical and also include Stryker and Mazor Robotics (now a part of Medtronic). Intuitive Surgical’s revenues were reported at $2.1 billion in the first six months of 2019, with 18 percent growth YOY. The da Vinci robot is now a fourth-generation system and priced at $1 million to $2 million per system. Intuitive had an installed base of 5,270 machines globally as of June 30, 2019, and 689 da Vinci robots were sold within Asia, with more than 100 in India.7

Table 2: Projected global growth of the surgical robotics market8
A report from the Royal College of Surgeons notes the changes in the beginning and ensuing adoption of robotic surgery over the past 20 years, which was created and dominated by the da Vinci surgical robotics platform.9 The paper describes the current commercially available systems and new, innovative systems in development. With a nod to the expense of robotics systems, the authors indicate a higher level of competition should result in reduced costs and improved market access. Less likely, the authors suggest, is collaboration between surgical robotic system manufacturers to standardize basic features, which would be desirable to surgeons, but would reduce differentiation and switching costs between systems. Several novel surgical robotics systems are in development for applications in gynecological, colorectal, cardiothoracic, upper GI, renal, urological, and general surgery.
Where’s the Growth Coming From?
As the use of surgical robotics increased, the Michigan study procedural trend saw a decline in laparoscopic surgery from 53.2 percent prior to adopting robotic surgery to 51.3 percent after the surgical robots were installed. In addition, growth drivers include: rising technological advancement in surgical robotic tools in developed countries, an increase in the number of procedures in North America and Asia Pacific, expanding healthcare spending in emerging countries, and a positive regulatory scenario and the aging of the baby boomer generation in the U.S.10
Don’t Forget the Adverse Events
Even with broad adoption of surgical robotic systems, adverse events (death, patient injuries, and device malfunctions) are still seen during procedures. Most are preventable incidents and need to be addressed in the design of surgical robotic platforms. During the study period, (2000-2013), 10,624 adverse events were reported (Table 3). Surprisingly, the number of injury and deaths per procedure have remained relatively constant over this period.11

Table 3: Adverse events (n=10,624) in robotic surgery from 2000-2013.11
The Medi-Vantage Perspective
Robots are here to stay, and robotic surgery will continue to develop as a critical surgical skill. The question is, which medtech company will have the ability to address the entire market, instead of smaller segments?
References
- bit.ly/mpo201001
- bit.ly/mpo201002
- bit.ly/mpo201003
- bit.ly/mpo201004
- bit.ly/mpo201005
- bit.ly/mpo201006
- bit.ly/mpo201007
- bit.ly/mpo201008
- bit.ly/mpo201009
- bit.ly/mpo201010
- bit.ly/mpo201011
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses, speaks regularly at medtech conferences, and can be reached at mshepherd@medi-vantage. Visit her website at www.medi-vantage.com.