Sean Hägen, Principal, Director of Research & Synthesis, BlackHägen Design09.01.20
Often the aesthetic of a device or system experience is considered a commercial objective and even a tertiary, superficial aspect of design development in the healthcare ecosystem. Human factors engineering (HFE) research has theorized the aesthetics of a device or user interface may actually influence its usability, that is, its apparent usability may be as important as actual usability.1 This discussion will specifically address the relationship between the aesthetic of a device, HFE, and how it can be purposefully designed to optimize use-safety and ease-of-use.
The process of a user understanding a device’s operation is a complex amalgamation of perceptional translation, biases, interactions, reasoning, and expectations that starts with an initial assessment. This process is especially critical when there is no product training or a significant lapse between use and training. In healthcare, the initial interaction with a device is often critical to a person’s well-being.
A collaborative team of at least HFE and industrial design (ID), utilizing the product semantics methodology as an overarching approach, can leverage purposely intentioned aesthetics that optimize usability by creating affordances to enable anticipated interaction. In other words, implement a process that proactively defines “intuitive” usability. Usability is not the only objective for ID, but it can drive most of the medical HFE process.
For the purpose of this discussion, we are going to focus on devices as opposed to other user experiences and UI such as environments and graphic user interfaces. The following definitions are relevant to the context of the discussion.
Aesthetic: Concerned with beauty or the appreciation of beauty. A set of principles or branch of philosophy that deals with the nature of beauty and taste. It examines subjective and sensori-emotional values, or sometimes called judgments of sentiment and taste .
It has been said that “beauty is intrinsic, because an object is perceived without any reasoning about expected utility,”2 perhaps because “reasoning” is primarily a conscious activity. However, beauty is initially assessed on a subconscious level, a visceral impression which, arguably, includes an associated relationship with utility.
Usability: Characteristics of the user interface that facilitate use and thereby establish effectiveness, efficiency, and user satisfaction in the intended use environment.2
For this discussion’s purpose, the standard IEC2 premise of usability is expanded on as a combination of two subsets—Ease-of-Use: A commercial perspective for the user convenience typically focused on marketing objectives; and Use-Safety: A regulatory perspective, focused on use-related risk management and life safety. The two aspects often result in contradicting requirements or conflicting features, so designing a solution that harmonizes these two elements of usability is challenging and ideal.
Product Semantics: A systematic inquiry into how people attribute meaning to [products] and interact with them accordingly3; and a vocabulary and methodology for designing [products] in view of the meaning they could acquire for their users and the communities of their stakeholders.3
A device’s form and aesthetic composition does not literally explain what it does; however, it is how the user interprets it.4 A goal of product semantics is to enable a self-evident user interface through the qualities of the product’s aesthetic, form, shape, texture, and color. Although product semantics is practiced primarily by industrial designers (ID), medical device design is commonly a cross-functional team activity where HFE, user experience designers, and others collaborate to develop the design language.
Affordances: A user’s perception of potential interaction with an object based on its properties are referred to as its affordances. In this capacity, it is a subset of product semantics.
Discussion
Designing for aesthetic appraisal and beyond to aesthetic judgment,2 that is, the initial impression to the entirety of the user experience, requires the design team to understand the context of use (including use-related risks) to prioritize how user needs translate into aesthetics and affordances.
This circles back to the harmonization between use-safety, ease-of-use, and delight. Often the driving requirements conflict, so it is essential to have a thorough understanding of the contextual origination of the needs that informed the requirements. Also, insights based on observation of users and their context of use should identify potential positive and negative transfer bias the designers have to consider when characterizing the design language’s attributes. “An object’s form first says something about the object itself, then secondarily something about the larger context of its use; both to the user who interacts with it and to the designer that develops those conceptual connections.”5
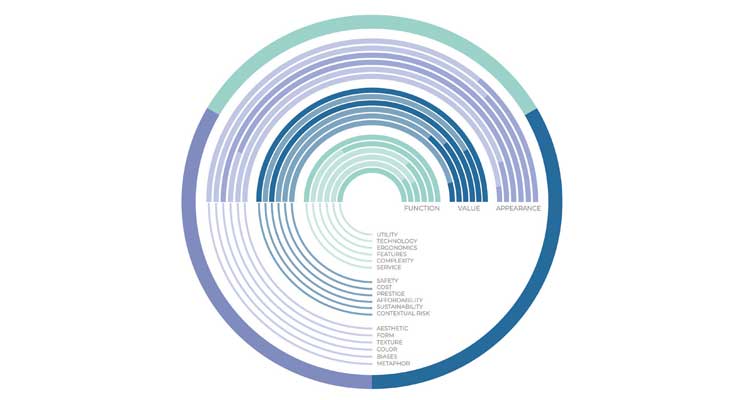
Regardless of how the process is executed, IDs need enough fidelity in the requirements that influence the aesthetic (beyond corporate identity) to address subjective attributes like “intuitive” and “easy.” This level of information is typically not available, and HFE is at a loss to add resolution to those requirements. Specifically, user interface requirements that will ultimately provide affordances that enable the user to intuitively experience their expected outcome, such that the critical tasks in particular, are self-evident. Prioritizing and expanding on perception attributes (Figure 1) and articulating the applicable attributes in the explanation of user interface (UI) requirements can guide in development of the appropriate product semantic.
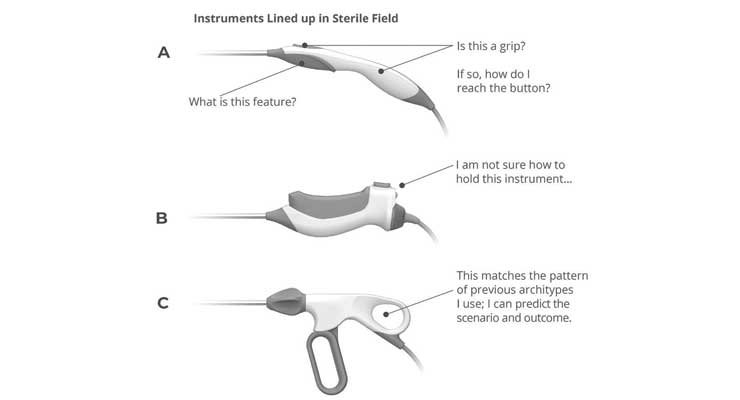
In the healthcare space the design team must consider all those attributes while also focusing even further on highly specialized use case scenarios. This must be done to mitigate use-related risks of harm to the patient and/or user while also providing an expected and pleasurable—or intentionally invisible—experience. The challenge is, how do you know how perception attributes are ranked by different user personas? There are likely multiple semantic schemes that meet the user needs information at the depth available, so which direction should be pursued (Figures 2&3)?
The answer is a user evaluation of configuration models, where the study can evaluate aesthetic assessment and judgment while probing feature preferences rather than a contest of individual design concepts, i.e., a user preference study.
In order to design in a systematic manner for a user’s first impression—or aesthetic appraisal5—to have a purposeful meaning, there are two key factors to inform the design process: initial user perception (Figure 1) and context of use (includes user needs).
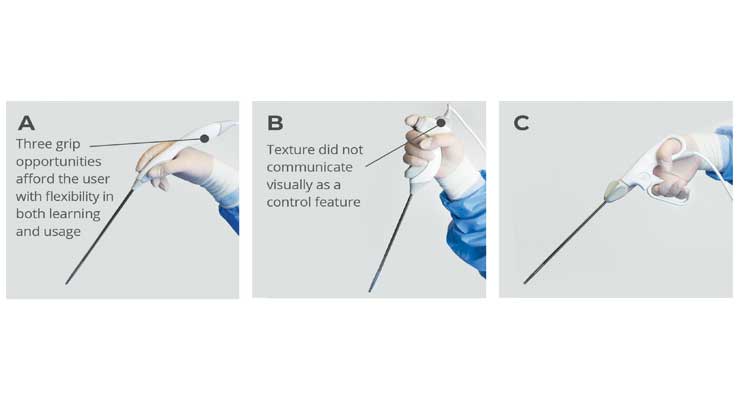
For example, a hypothetical scenario with a physician in a clinical environment (assuming the user was not involved in the devices’ purchasing process). Three new devices are laid out as they would be on an instrument table (Figure 2). These types of devices typically do not require formal training, a brief informal “in-service” will likely be all they receive. The user’s prior experience with other devices and their grip configurations introduces negative transfer bias during the initial assessment of affordances, and later during haptic discovery (Figure 3).
If the goal of this new design is to change the user’s grip to promote a neutral wrist orientation during surgery, conventional designs may not provide. A radical deviation from the user’s learned context will potentially compromise intuitive interaction. Strategies to address this include either a plan that evolves the user experience (UX) and grip design over the course of model releases, or a UI that accommodates both the legacy UI and a new design (Figure 2-A). The latter approach, if executed with a semantic methodology, should accommodate the context of use such that the UI offers affordances for multiple needs, rather than prescribing a “correct” way to interact with the device.
A common challenge usability engineering specialists have characterizing user needs and UI requirements is trying to understand and characterize intuitive use, or worse yet, “ease of use.” The approach of product semantics, which includes affordances,6 enables utilizing the visceral impact of a device’s aesthetic to communicate interaction cues, including but not limited to form, shape, texture, color, and metaphors.
For example, a patient’s initial subliminal impression of an imaging machine may impact their vital signs based on an experience where the device’s aesthetic plays a dominant role.6 The use of subtle metaphor can set conscious and subconscious expectations to create a more relaxed experience with an imaging system.
Conclusion
Product semantics is a model that not only relates the aspects of design, but also harmonizes them into a cohesive, repeatable methodology. “Through product semantics, designers can demystify complex technology, improve the interaction between [products] and their users and enhance opportunities for self-expression.”4
Looking backwards from validation in the HFE process: Formative studies typically identify tasks that have usability issues and many are characterized as not “intuitive,” requiring the design to be more resilient and “user-friendly.” Those user evaluations are purposely designed so the user is not coached on how the device operates to expose usability issues and, by default, demonstrate intuitiveness. When the HFE analysts report on usability issues, including subjective inputs from the participants, there is language used to describe the deficiencies and/or infelicities. These can be starting places for understanding the perceived attributes that inform the product semantics.
Consider product semantics a design language for optimizing usability, where the syntax is the affordances—aesthetic, shape, form, color, and metaphor; and the vocabulary is its perceived attributes informed from the HFE process and context of use.
References
Since founding BlackHägen Design (BH) in 1995, Sean has been the principal investigator for design research, within both institutional and home environments, across 20 countries. Sean has published numerous articles on usability research and design, as well as contributed to domestic and international standards for usability development processes, as a member of the AAMI HE committee (ANSI HE75:2009 and IEC 62366-1:2015). In this role, he is a contributing author for AAMI TIR 50 and 59 as well as the revision of ANSI/AAMI HE75. He was the IDSA Medical Section Chair for eight years and sat on the Board of Directors for two terms. He currently chairs the IDSA Patient Safety Task Force.
The process of a user understanding a device’s operation is a complex amalgamation of perceptional translation, biases, interactions, reasoning, and expectations that starts with an initial assessment. This process is especially critical when there is no product training or a significant lapse between use and training. In healthcare, the initial interaction with a device is often critical to a person’s well-being.
A collaborative team of at least HFE and industrial design (ID), utilizing the product semantics methodology as an overarching approach, can leverage purposely intentioned aesthetics that optimize usability by creating affordances to enable anticipated interaction. In other words, implement a process that proactively defines “intuitive” usability. Usability is not the only objective for ID, but it can drive most of the medical HFE process.
For the purpose of this discussion, we are going to focus on devices as opposed to other user experiences and UI such as environments and graphic user interfaces. The following definitions are relevant to the context of the discussion.
Aesthetic: Concerned with beauty or the appreciation of beauty
It has been said that “beauty is intrinsic, because an object is perceived without any reasoning about expected utility,”2 perhaps because “reasoning” is primarily a conscious activity. However, beauty is initially assessed on a subconscious level, a visceral impression which, arguably, includes an associated relationship with utility.
Usability: Characteristics of the user interface that facilitate use and thereby establish effectiveness, efficiency, and user satisfaction in the intended use environment.2
For this discussion’s purpose, the standard IEC2 premise of usability is expanded on as a combination of two subsets—Ease-of-Use: A commercial perspective for the user convenience typically focused on marketing objectives; and Use-Safety: A regulatory perspective, focused on use-related risk management and life safety. The two aspects often result in contradicting requirements or conflicting features, so designing a solution that harmonizes these two elements of usability is challenging and ideal.
Product Semantics: A systematic inquiry into how people attribute meaning to [products] and interact with them accordingly3; and a vocabulary and methodology for designing [products] in view of the meaning they could acquire for their users and the communities of their stakeholders.3
A device’s form and aesthetic composition does not literally explain what it does; however, it is how the user interprets it.4 A goal of product semantics is to enable a self-evident user interface through the qualities of the product’s aesthetic, form, shape, texture, and color. Although product semantics is practiced primarily by industrial designers (ID), medical device design is commonly a cross-functional team activity where HFE, user experience designers, and others collaborate to develop the design language.
Affordances: A user’s perception of potential interaction with an object based on its properties are referred to as its affordances. In this capacity, it is a subset of product semantics.
Discussion
Designing for aesthetic appraisal and beyond to aesthetic judgment,2 that is, the initial impression to the entirety of the user experience, requires the design team to understand the context of use (including use-related risks) to prioritize how user needs translate into aesthetics and affordances.
This circles back to the harmonization between use-safety, ease-of-use, and delight. Often the driving requirements conflict, so it is essential to have a thorough understanding of the contextual origination of the needs that informed the requirements. Also, insights based on observation of users and their context of use should identify potential positive and negative transfer bias the designers have to consider when characterizing the design language’s attributes. “An object’s form first says something about the object itself, then secondarily something about the larger context of its use; both to the user who interacts with it and to the designer that develops those conceptual connections.”5
Regardless of how the process is executed, IDs need enough fidelity in the requirements that influence the aesthetic (beyond corporate identity) to address subjective attributes like “intuitive” and “easy.” This level of information is typically not available, and HFE is at a loss to add resolution to those requirements. Specifically, user interface requirements that will ultimately provide affordances that enable the user to intuitively experience their expected outcome, such that the critical tasks in particular, are self-evident. Prioritizing and expanding on perception attributes (Figure 1) and articulating the applicable attributes in the explanation of user interface (UI) requirements can guide in development of the appropriate product semantic.
In the healthcare space the design team must consider all those attributes while also focusing even further on highly specialized use case scenarios. This must be done to mitigate use-related risks of harm to the patient and/or user while also providing an expected and pleasurable—or intentionally invisible—experience. The challenge is, how do you know how perception attributes are ranked by different user personas? There are likely multiple semantic schemes that meet the user needs information at the depth available, so which direction should be pursued (Figures 2&3)?
The answer is a user evaluation of configuration models, where the study can evaluate aesthetic assessment and judgment while probing feature preferences rather than a contest of individual design concepts, i.e., a user preference study.
In order to design in a systematic manner for a user’s first impression—or aesthetic appraisal5—to have a purposeful meaning, there are two key factors to inform the design process: initial user perception (Figure 1) and context of use (includes user needs).
For example, a hypothetical scenario with a physician in a clinical environment (assuming the user was not involved in the devices’ purchasing process). Three new devices are laid out as they would be on an instrument table (Figure 2). These types of devices typically do not require formal training, a brief informal “in-service” will likely be all they receive. The user’s prior experience with other devices and their grip configurations introduces negative transfer bias during the initial assessment of affordances, and later during haptic discovery (Figure 3).
If the goal of this new design is to change the user’s grip to promote a neutral wrist orientation during surgery, conventional designs may not provide. A radical deviation from the user’s learned context will potentially compromise intuitive interaction. Strategies to address this include either a plan that evolves the user experience (UX) and grip design over the course of model releases, or a UI that accommodates both the legacy UI and a new design (Figure 2-A). The latter approach, if executed with a semantic methodology, should accommodate the context of use such that the UI offers affordances for multiple needs, rather than prescribing a “correct” way to interact with the device.
A common challenge usability engineering specialists have characterizing user needs and UI requirements is trying to understand and characterize intuitive use, or worse yet, “ease of use.” The approach of product semantics, which includes affordances,6 enables utilizing the visceral impact of a device’s aesthetic to communicate interaction cues, including but not limited to form, shape, texture, color, and metaphors.
For example, a patient’s initial subliminal impression of an imaging machine may impact their vital signs based on an experience where the device’s aesthetic plays a dominant role.6 The use of subtle metaphor can set conscious and subconscious expectations to create a more relaxed experience with an imaging system.
Conclusion
Product semantics is a model that not only relates the aspects of design, but also harmonizes them into a cohesive, repeatable methodology. “Through product semantics, designers can demystify complex technology, improve the interaction between [products] and their users and enhance opportunities for self-expression.”4
Looking backwards from validation in the HFE process: Formative studies typically identify tasks that have usability issues and many are characterized as not “intuitive,” requiring the design to be more resilient and “user-friendly.” Those user evaluations are purposely designed so the user is not coached on how the device operates to expose usability issues and, by default, demonstrate intuitiveness. When the HFE analysts report on usability issues, including subjective inputs from the participants, there is language used to describe the deficiencies and/or infelicities. These can be starting places for understanding the perceived attributes that inform the product semantics.
Consider product semantics a design language for optimizing usability, where the syntax is the affordances—aesthetic, shape, form, color, and metaphor; and the vocabulary is its perceived attributes informed from the HFE process and context of use.
References
- Kossack, M., Gellatly, A., & Jandrisits, A. (2007). Industrial Design and Human Factors: Design Synergy for Medical Devices. Human Computer Interaction International 2007, Beijing, P.R. China.
- IEC 62366-1:2015 Medical Devices – Part 1: Application of usability engineering medical devices. AAMI, 16 (3.16)
- Krippendorff, K. (2005). The Semantic Turn: A New Foundation for Design. CRC Press, 2,142
- Krippendorff, K., & Butter, R. (1984). Product semantics: Exploring the symbolic qualities of form. Innovation, 3(2), 4-9.
- Moshagen, M. & Thielsch, M. T. (2010). Facets of visual aesthetics. International Journal of Human-Computer Studies, 68(10), 689-709. doi:10.1016/j.ijhcs.2010.05.006
- Kleiss, J. A. (2016), Preliminary Development of a Multidimensional Semantic Patient Experience Measurement Questionnaire. Health Environments Research & Design Journal, (1-13) DOI: 10.1177/1937586716636841
Since founding BlackHägen Design (BH) in 1995, Sean has been the principal investigator for design research, within both institutional and home environments, across 20 countries. Sean has published numerous articles on usability research and design, as well as contributed to domestic and international standards for usability development processes, as a member of the AAMI HE committee (ANSI HE75:2009 and IEC 62366-1:2015). In this role, he is a contributing author for AAMI TIR 50 and 59 as well as the revision of ANSI/AAMI HE75. He was the IDSA Medical Section Chair for eight years and sat on the Board of Directors for two terms. He currently chairs the IDSA Patient Safety Task Force.