Helin Räägel, Ph.D., Biocompatibility Expert, Nelson Laboratories09.01.20
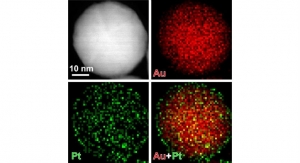
Medical devices have become increasingly complex over time and incorporation of “smart” features has become commonplace. These improvements may increase effectiveness of implantation, drug release, targeting specific organs, or data transmission from an implant, among many other possibilities. For example, a number of drug-delivery devices have been reduced in size to nanoscale. These nano-objects or nanoparticles (NP) are defined as a discrete piece of material with one, two, or three external dimensions ranging from 1 nm to 1 µm, and have properties or functionality enabled by their small scale. Their smaller size allows for the utilization of less invasive procedures to, for example, distribute a drug that is encapsulated within the NPs to a desired site of action. Depending on the composition of the NP, more regulated release of the drug (slower and more uniform over time) may be achieved, increasing the efficiency of the therapeutic treatment.
Despite all the benefits nanotechnology is designed to provide, however, its application to clinical human therapy has faced challenges as research on nano-therapy is relatively recent, and the risks associated with this type of treatment and the impact to the organism as a whole or to specific organs remains mostly uncharacterized and thus, unknown. For instance, due to their small size, NPs are also readily taken up by cells within the tissues, expanding the unknowns of local reactivity with, for example, proteins, nucleic acid, or cellular organelles. The situation is further complicated because each specific formulation of NPs may have different kinetics of absorption, distribution, metabolism, and excretion (ADME). So how should we assess medical devices for biocompatibility that are either containing or entirely composed of nanoscale particles?
As with any medical device, ISO 10993-1 Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process highlights that the process should begin with a biological risk evaluation, which includes assessment of the specific patient risks and benefits upon use of the given device. This is also highlighted in ISO 14971 Medical devices – Application of risk management to medical devices, which states “the decision to use a medical device in the context of a particular clinical procedure requires the residual risks to be balanced against the anticipated benefits of the procedure.” Further, it explains such judgments should “take into account the intended use, performance, and risks associated with the medical device, as well as the risks and benefits associated with the clinical procedure or the circumstances of use.” With the elevated risks of nano-sized medical devices or combination products (nano-devices designed and intended to be used with drugs), this initial risk analysis is more crucial as they inherently carry higher risks, a lack of clinical experience compared to other device types, and are typically less rigorously studied from the perspective of biocompatibility. ISO 10993-1 also specifies nanomaterials require specific attention, because materials with sub-micron components have been shown, in some cases, to behave differently than the same materials at larger scales, and due to this, the extrapolation of data from larger sized materials is not appropriate. In addition, the specific physical form (geometry, particle size, porosity, surface texture) can have a substantial effect on biological interactions and in fine impact safety. Thus, consider these aspects as part of the overall risk evaluation.
Some guidance on what to specifically focus on when dealing with nanoscale materials is provided in ISO/TR 10993-22 Biological evaluation of medical devices — Part 22: Guidance on nanomaterials. ISO/TR 10993-22 is intended to provide a general framework for the assessment of NPs or NP-containing or -generating medical devices, and highlights the important aspects that need to be considered. It defines the common framework proposed in ISO 10993-1 Annex A is also generally applicable for these types of medical devices, and the risk management process (which includes hazard identification, exposure assessment, and risk estimation) “is generally sufficiently robust and flexible to provide a basis for evaluation of nanomaterials, even though they can have properties that can be different from conventional ones.” However, as stated previously, additional risks due to their size should be considered as well.
Table 1 of ISO 10993-22 gives various examples of the typical types of nanomaterials used in medical devices and specifies the considerations (in addition to the ISO 10993-1 evaluation) that should be addressed, such as characterization of physicochemical properties, toxicokinetics, tissue distribution, and so forth. Furthermore, it should be taken into consideration that the NPs will most likely be distributed within the body using the circulatory and/or the lymphatic system where they (due to their size) are expected to be engulfed by cells of the mononuclear phagocytic system (MPS; macrophages, dendritic cells, or Langerhans cells). These cells play a central role in the immune system, therefore, the potential immunotoxicity of NPs needs specific consideration in the overall risk assessment.
A European Commission white paper on the regulatory approach to take for nanotechnology-enabled health products states “Identification of the major proteins that will bind to NP surface and the amount of proteins that will bind over time can give some insights into the circulation time, biodistribution, and risk of the immunological effects.”1 The paper also discusses that while immunotoxicity is not automatically tested for all medicinal products and can be difficult to assess due to a multitude of factors such as species-dependent reactions, lack of common test methods, and so on, if present, it will likely be observed in standard toxicity studies as an alteration in routinely tested parameters such as blood chemistry, blood cell count, and others. These can, in general, be considered good initial indicators of any risk of adverse immune effects. Thus, if any previous subacute/subchronic animal studies have been performed on the NPs, while inter-species variability may exist, review and analyze the data from these studies to identify whether the immunogenicity is a clinically relevant concern for the specific NPs.1
The white paper also highlights other additional endpoints relevant for nanotechnology-based medical products, such as stability in (as well as compatibility with) blood and serum, biological fate, accumulation issues, ADME, in-vitro uptake and cytotoxicity to phagocytes, in-vivo degradation, etc. U.S. FDA guidance also indicates “nanomaterials entering the blood circulation interact with multiple plasma proteins in a process lasting over several hours, which ultimately results in the formation of a protein corona” which may endow the NPs with new biological properties.2 Further, unique properties indicated in the FDA draft guidance document include aggregation, and agglomeration, as well as the previously indicated immunogenicity, or toxicity. It also emphasizes that nanomaterials may require “specialized techniques, if characterization and biocompatibility testing is needed.”2
While ISO 10993-22, the European Commission white paper, and the U.S. FDA draft guidance document on NPs provide a general basis and thought process for performing the initial risk assessment for NPs, none of them outline or provide detailed testing protocols. On one hand, it is understandable, as each NP has a specific set of characteristics hard to “squeeze” into a standard approach of testing. For instance, NPs containing iron can interfere with typical in-vitro test systems used to assess hemocompatibility studies, as hemolysis tests per ASTM F756-17 Standard practice for assessment of hemolytic properties of materials measure the hemoglobin concentration based on the absorptivity of the sample’s iron content. Additionally, standard implantation testing per ISO 10993-6 Biological evaluation of medical devices – Part 6: Tests for local effects after implantation might not be feasible for NPs as they are not expected to remain at the injection site but rather disperse rapidly to secondary locations in the body; furthermore, also due to their size, the NPs are not easily recognized in the specific tissues, making identification and tissue reactivity assessment using standard methods challenging. In light of this, a more extensive systemic toxicity study with the weight and pathology of the principal body organs to evaluate the effect of the NPs on the micro-structure of the tissues can be considered a more appropriate option to address the implantation endpoint.
Additionally, specific parameters or exposure routes used in the set of recommended tests should be critically evaluated to ensure appropriate conditions are utilized in the studies. For instance, if per the instructions for use, the NPs should not be injected intravenously (IV) to the patient, IV exposure in the test animals should also be avoided as it may cause clinically irrelevant toxicity profiles in the studies. In this particular case, since some ISO 10993 series tests utilize the IV route as a standard administration method to the test animals (for example systemic toxicity and material mediated pyrogenicity), these should be tailored to the specific use of the NPs or omitted entirely due to the potential for incompatibility. However, if an endpoint is omitted from the testing plan, assessment using other means or available data should be performed. All these aspects further highlight the importance of having a thorough biological evaluation plan performed as an initial step of defining the testing approach needed to verify biocompatibility for NPs. Moreover, in cases where the NPs are intended to be used as drug delivery platforms, attention should also be paid to the compatibility between the drug and the NPs, as well as the efficiency and release profile of the drug. Since the inclusion of a drug may also affect the ADME of the NPs, this may need to be investigated or verified further as well.
While the lack of guidance essentially gives the freedom to adapt the testing plan and approach to the NPs’ specific needs, the true downside of not having a standardized testing platform is it leaves the door open for various potential types of approaches. This can come under scrutiny during the submission process with a regulatory body, and require additional justification effort. This further highlights the importance of performing a rigorous scientific initial risk assessment, in the form of a biological evaluation plan (BEP) as the first step that would define the reasoning and justification for the specific choice of tests and parameters. This BEP should also summarize all the currently available information on the product and include references to associated published literature or any internal investigational studies or research performed on the NPs. This review will assist in identifying and reducing some of the specific risks that can be associated with the nano-devices. This includes any knowledge on the specific chemicals or compounds used in the composition of the NPs, their toxicokinetics, degradation patterns, targeting within the body, and elimination from the body.
To use existing data for the biological evaluation, demonstration of nanomaterial equivalence is necessary. However, as nanomaterials carry such high inherent risk, ISO 10993-22 specifies in clause 4.4. that “in general, extrapolation of results by using existing data from other products using/containing similar nanomaterials, or from the corresponding parent compound of the same substance is not applicable, although such products can give an indication of possible safety concerns. If testing is considered necessary, it should be performed on the actual product and/or any nanomaterials which can come into contact with patients.”
In summary, for NPs or devices containing nanomaterials, the development of a biological evaluation plan as an initial risk assessment (although always recommended as a primary stage for any type of devices) is vital to accurately assess the biological risks associated with them. Also, due to the very custom nature of the approaches used to verify the biocompatibility of the nano-objects, it is always highly recommended to pursue up-front regulatory body feedback on the approach outlined in the plan (e.g., through a Pre-Sub submission with the U.S. FDA), in order to receive input and any additional guidance/concerns before initiating the testing.
References
Dr. Helin Räägel is an expert in biocompatibility, especially with respect to the relationship between medical device materials, biological systems, and the analytical procedures used to evaluate biocompatibility in-vitro and in-vivo. She has over 10 years of experience in scientific research, over two years of medical device experience managing validation work for regulatory acceptance, and is currently working as a biocompatibility expert in the technical consulting group at Nelson Labs.
Despite all the benefits nanotechnology is designed to provide, however, its application to clinical human therapy has faced challenges as research on nano-therapy is relatively recent, and the risks associated with this type of treatment and the impact to the organism as a whole or to specific organs remains mostly uncharacterized and thus, unknown. For instance, due to their small size, NPs are also readily taken up by cells within the tissues, expanding the unknowns of local reactivity with, for example, proteins, nucleic acid, or cellular organelles. The situation is further complicated because each specific formulation of NPs may have different kinetics of absorption, distribution, metabolism, and excretion (ADME). So how should we assess medical devices for biocompatibility that are either containing or entirely composed of nanoscale particles?
As with any medical device, ISO 10993-1 Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process highlights that the process should begin with a biological risk evaluation, which includes assessment of the specific patient risks and benefits upon use of the given device. This is also highlighted in ISO 14971 Medical devices – Application of risk management to medical devices, which states “the decision to use a medical device in the context of a particular clinical procedure requires the residual risks to be balanced against the anticipated benefits of the procedure.” Further, it explains such judgments should “take into account the intended use, performance, and risks associated with the medical device, as well as the risks and benefits associated with the clinical procedure or the circumstances of use.” With the elevated risks of nano-sized medical devices or combination products (nano-devices designed and intended to be used with drugs), this initial risk analysis is more crucial as they inherently carry higher risks, a lack of clinical experience compared to other device types, and are typically less rigorously studied from the perspective of biocompatibility. ISO 10993-1 also specifies nanomaterials require specific attention, because materials with sub-micron components have been shown, in some cases, to behave differently than the same materials at larger scales, and due to this, the extrapolation of data from larger sized materials is not appropriate. In addition, the specific physical form (geometry, particle size, porosity, surface texture) can have a substantial effect on biological interactions and in fine impact safety. Thus, consider these aspects as part of the overall risk evaluation.
Some guidance on what to specifically focus on when dealing with nanoscale materials is provided in ISO/TR 10993-22 Biological evaluation of medical devices — Part 22: Guidance on nanomaterials. ISO/TR 10993-22 is intended to provide a general framework for the assessment of NPs or NP-containing or -generating medical devices, and highlights the important aspects that need to be considered. It defines the common framework proposed in ISO 10993-1 Annex A is also generally applicable for these types of medical devices, and the risk management process (which includes hazard identification, exposure assessment, and risk estimation) “is generally sufficiently robust and flexible to provide a basis for evaluation of nanomaterials, even though they can have properties that can be different from conventional ones.” However, as stated previously, additional risks due to their size should be considered as well.
Table 1 of ISO 10993-22 gives various examples of the typical types of nanomaterials used in medical devices and specifies the considerations (in addition to the ISO 10993-1 evaluation) that should be addressed, such as characterization of physicochemical properties, toxicokinetics, tissue distribution, and so forth. Furthermore, it should be taken into consideration that the NPs will most likely be distributed within the body using the circulatory and/or the lymphatic system where they (due to their size) are expected to be engulfed by cells of the mononuclear phagocytic system (MPS; macrophages, dendritic cells, or Langerhans cells). These cells play a central role in the immune system, therefore, the potential immunotoxicity of NPs needs specific consideration in the overall risk assessment.
A European Commission white paper on the regulatory approach to take for nanotechnology-enabled health products states “Identification of the major proteins that will bind to NP surface and the amount of proteins that will bind over time can give some insights into the circulation time, biodistribution, and risk of the immunological effects.”1 The paper also discusses that while immunotoxicity is not automatically tested for all medicinal products and can be difficult to assess due to a multitude of factors such as species-dependent reactions, lack of common test methods, and so on, if present, it will likely be observed in standard toxicity studies as an alteration in routinely tested parameters such as blood chemistry, blood cell count, and others. These can, in general, be considered good initial indicators of any risk of adverse immune effects. Thus, if any previous subacute/subchronic animal studies have been performed on the NPs, while inter-species variability may exist, review and analyze the data from these studies to identify whether the immunogenicity is a clinically relevant concern for the specific NPs.1
The white paper also highlights other additional endpoints relevant for nanotechnology-based medical products, such as stability in (as well as compatibility with) blood and serum, biological fate, accumulation issues, ADME, in-vitro uptake and cytotoxicity to phagocytes, in-vivo degradation, etc. U.S. FDA guidance also indicates “nanomaterials entering the blood circulation interact with multiple plasma proteins in a process lasting over several hours, which ultimately results in the formation of a protein corona” which may endow the NPs with new biological properties.2 Further, unique properties indicated in the FDA draft guidance document include aggregation, and agglomeration, as well as the previously indicated immunogenicity, or toxicity. It also emphasizes that nanomaterials may require “specialized techniques, if characterization and biocompatibility testing is needed.”2
While ISO 10993-22, the European Commission white paper, and the U.S. FDA draft guidance document on NPs provide a general basis and thought process for performing the initial risk assessment for NPs, none of them outline or provide detailed testing protocols. On one hand, it is understandable, as each NP has a specific set of characteristics hard to “squeeze” into a standard approach of testing. For instance, NPs containing iron can interfere with typical in-vitro test systems used to assess hemocompatibility studies, as hemolysis tests per ASTM F756-17 Standard practice for assessment of hemolytic properties of materials measure the hemoglobin concentration based on the absorptivity of the sample’s iron content. Additionally, standard implantation testing per ISO 10993-6 Biological evaluation of medical devices – Part 6: Tests for local effects after implantation might not be feasible for NPs as they are not expected to remain at the injection site but rather disperse rapidly to secondary locations in the body; furthermore, also due to their size, the NPs are not easily recognized in the specific tissues, making identification and tissue reactivity assessment using standard methods challenging. In light of this, a more extensive systemic toxicity study with the weight and pathology of the principal body organs to evaluate the effect of the NPs on the micro-structure of the tissues can be considered a more appropriate option to address the implantation endpoint.
Additionally, specific parameters or exposure routes used in the set of recommended tests should be critically evaluated to ensure appropriate conditions are utilized in the studies. For instance, if per the instructions for use, the NPs should not be injected intravenously (IV) to the patient, IV exposure in the test animals should also be avoided as it may cause clinically irrelevant toxicity profiles in the studies. In this particular case, since some ISO 10993 series tests utilize the IV route as a standard administration method to the test animals (for example systemic toxicity and material mediated pyrogenicity), these should be tailored to the specific use of the NPs or omitted entirely due to the potential for incompatibility. However, if an endpoint is omitted from the testing plan, assessment using other means or available data should be performed. All these aspects further highlight the importance of having a thorough biological evaluation plan performed as an initial step of defining the testing approach needed to verify biocompatibility for NPs. Moreover, in cases where the NPs are intended to be used as drug delivery platforms, attention should also be paid to the compatibility between the drug and the NPs, as well as the efficiency and release profile of the drug. Since the inclusion of a drug may also affect the ADME of the NPs, this may need to be investigated or verified further as well.
While the lack of guidance essentially gives the freedom to adapt the testing plan and approach to the NPs’ specific needs, the true downside of not having a standardized testing platform is it leaves the door open for various potential types of approaches. This can come under scrutiny during the submission process with a regulatory body, and require additional justification effort. This further highlights the importance of performing a rigorous scientific initial risk assessment, in the form of a biological evaluation plan (BEP) as the first step that would define the reasoning and justification for the specific choice of tests and parameters. This BEP should also summarize all the currently available information on the product and include references to associated published literature or any internal investigational studies or research performed on the NPs. This review will assist in identifying and reducing some of the specific risks that can be associated with the nano-devices. This includes any knowledge on the specific chemicals or compounds used in the composition of the NPs, their toxicokinetics, degradation patterns, targeting within the body, and elimination from the body.
To use existing data for the biological evaluation, demonstration of nanomaterial equivalence is necessary. However, as nanomaterials carry such high inherent risk, ISO 10993-22 specifies in clause 4.4. that “in general, extrapolation of results by using existing data from other products using/containing similar nanomaterials, or from the corresponding parent compound of the same substance is not applicable, although such products can give an indication of possible safety concerns. If testing is considered necessary, it should be performed on the actual product and/or any nanomaterials which can come into contact with patients.”
In summary, for NPs or devices containing nanomaterials, the development of a biological evaluation plan as an initial risk assessment (although always recommended as a primary stage for any type of devices) is vital to accurately assess the biological risks associated with them. Also, due to the very custom nature of the approaches used to verify the biocompatibility of the nano-objects, it is always highly recommended to pursue up-front regulatory body feedback on the approach outlined in the plan (e.g., through a Pre-Sub submission with the U.S. FDA), in order to receive input and any additional guidance/concerns before initiating the testing.
References
Dr. Helin Räägel is an expert in biocompatibility, especially with respect to the relationship between medical device materials, biological systems, and the analytical procedures used to evaluate biocompatibility in-vitro and in-vivo. She has over 10 years of experience in scientific research, over two years of medical device experience managing validation work for regulatory acceptance, and is currently working as a biocompatibility expert in the technical consulting group at Nelson Labs.