Paul Boentges and Sam Ramnarine, Jabil Healthcare03.04.20
Ethylene oxide (EO) is a flammable, colorless gas used in the healthcare manufacturing industry for medical device sterilization. It is also used to make other chemicals that are the building blocks for a range of non-healthcare products, including antifreeze, textiles, plastics, detergents, and adhesives. It is an important chemical in our society.
For decades, EO has been successfully used as a terminal sterilization method for medical devices that cannot be adequately sterilized by other sterilization methods such as a radiation sterilization (gamma, e-beam, and X-ray), moist-heat sterilization (steam), dry-heat sterilization, vaporized hydrogen peroxide sterilization (VPA), and gas plasma sterilization (STERRAD).
Most major medical device manufacturers use EO as the primary method of terminal sterilization utilizing in-house capabilities or contract sterilization service providers such as Steris and Sterigenics. Approximately 50 percent of all medical devices are sterilized utilizing EO.
The Background
Ethylene oxide is one of 187 hazardous air pollutants or “air toxics” the EPA regulates under the Clean Air Act, placed initially on the federal list of carcinogens in 1985. In 2016, the EPA released a reassessment linking the chemical to diseases such as breast cancer, leukemia, and lymphomas. In addition to updating its risk value for ethylene oxide, the EPA has committed to working with state, local, and tribal air agencies to address this chemical.
In February 2019, the Sterigenics EO contract sterilization facility in Willowbrook, Ill., was ordered to cease operations as a result of air testing performed by the EPA’s Illinois branch office. Shortly thereafter, the Illinois legislature signed into law the nation’s strictest limits on the emission of the cancer-causing chemical (EO) as a result of an investigation into the relationship between emissions from the Sterigenics’ Willowbrook facility and the high rates of cancer in the surrounding area. As a result, Illinois legislation now mandates companies using EO must be completely contained within facilities and the results of annual testing must be submitted to the Illinois EPA. Consequently, Sterigenics has communicated it will no longer offer EO contract sterilization services at the Willowbrook facility.
Impact on Contract Sterilization Facility Volumes
According to the FDA’s Establishment Registration & Device Listing database, Sterigenics listed a total of 594 types of devices that underwent terminal EO sterilization in Willowbrook. These products included sutures, clamps, knives, stents, needles, and many other medical devices. It is estimated the Willowbrook facility could have represented as much as five percent of the company’s total EO volume.
As a result of the closure, medical device manufacturers have been aggressively working on identifying and qualifying alternate EO sterilization sites within the United States. Other states have initiated similar evaluations with sterilization sites that utilize EO as a sterilization agent. These sites include the Sterigenics EO contract sterilizer in Smyrna, Ga.; the Becton Dickinson in-house EO sterilizer in Covington, Ga.; the Viant Medical in-house EO sterilizer in Grand Rapids, Mich.; and the Medline EO in-house sterilizer in Mundelein, Ill.
FDA’s Shortages Response
Shutdowns of several EO sterilization sites have raised concerns about potential shortages in the healthcare industry since one half of all medical devices are sterilized using ethylene oxide. In response to the recent closure of several EO sterilization facilities over environmental concerns, the U.S. Food and Drug Administration (FDA) is taking aggressive steps to ensure hospitals, healthcare providers, and patients have access to medical devices that are safely and effectively sterilized.
Two federal agencies—the EPA and the FDA—have now both become collaborative stakeholders in managing this issue, but from different perspectives: reducing exposure to toxic emissions vs. ensuring critical medical device availability.
The FDA maintains a page on its website—Ethylene Oxide Sterilization Facility Updates1—with the sole purpose of providing timely information on any future actions the agency takes regarding medical device shortages and other activities associated with this issue. The following statement appears at the top of the page:
“The FDA is closely monitoring the supply chain effects of closures and potential closures of certain facilities that use ethylene oxide to sterilize medical devices prior to their use. The Agency is concerned about the future availability of sterile medical devices and the potential for medical device shortages that might impact patient care.”
FDA’s Innovation Challenges
On July 15, 2019, the FDA announced2 the launch of two public innovation challenges to encourage innovation in medical device sterilization—one focused on reducing emissions and the other encouraging alternative sterilization methods.
Challenge 1 is focused on identifying alternatives to EO sterilization methods. Table 1 lists the four participants selected from 24 applications.3

Table 1: The four participants selected from 24 applications to compete in the FDA’s challenge to identify alternatives to EO sterilization for medical devices.3
Challenge 2 is focused on developing strategies to reduce EO emissions. The FDA received 22 applications and selected eight participants (Table 2).4
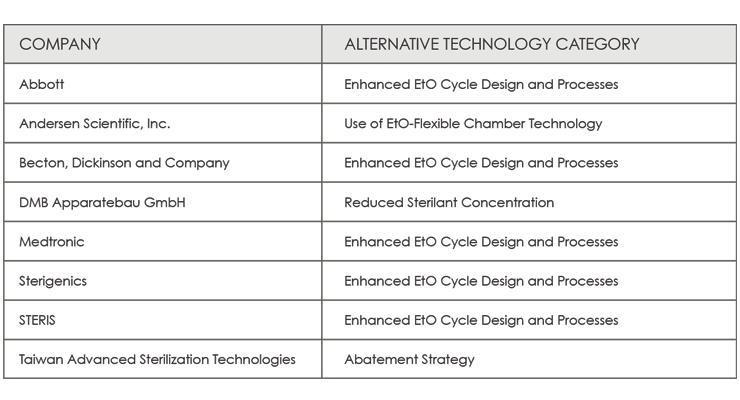
Table 2: The eight participants selected from 22 applications to compete in the FDA’s challenge to develop strategies to reduce EO emissions.4
The FDA’s Center for Devices and Radiological Health encouraged participation in these challenges from organization such as medical devices companies, distributors, technology manufacturers, laboratories, academic institutions, healthcare facilities, professional societies, foundations, and not-for-profits. Based on current knowledge, medical device manufacturers (Johnson & Johnson, Becton Dickinson), sterilization equipment manufacturers, academic institutions, and professional societies (AAMI, AdvaMed) submitted responses to the FDA Challenge initiative.
Alternative Sterilization Method Feasibility
To proactively address potential product availability, as well as supply chain impacts, medical device manufacturers have launched internal product conversion initiatives to determine the feasibility of qualifying alternate sterilization methods for medical devices. Currently, radiation sterilization (gamma, e-beam, and X-ray) is the only feasible alternative due to its availability and long history of safe use within the healthcare industry. Unfortunately, many medical devices are not compatible with radiation sterilization due to product functionality limitations when exposed to radiation and its consequential degradative effects on materials (e.g., cross-linking, discoloration of plastics, embrittlement, etc.).
Of the three radiation sterilization modalities, gamma radiation utilizing cobalt-60 is the most prevalent. In North America, however, there is only one source for cobalt-60: Nordion, a subsidiary of Sotera Health. Demand for cobalt-60 in all radiation processing applications has been increasing in recent years (90 percent is used for the sterilization of disposable medical products, 5 percent for food irradiation, and 5 percent for other applications; Table 3) and will continue to ramp up over the next one to two years as more medical device manufacturers qualify products for terminal sterilization utilizing gamma radiation.
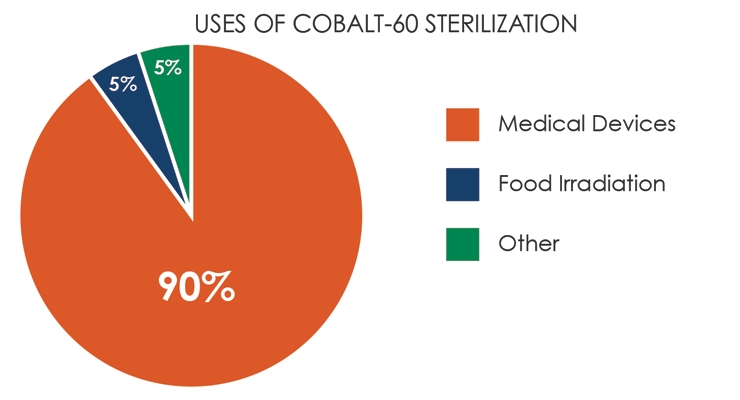
Table 3: Demand for cobalt-60 in all radiation processing applications has been increasing in recent years.
Another important factor to consider: since cobalt-60 decays continuously, supply of the isotope will be under additional pressure as existing sites currently providing gamma sterilization [e.g., Jabil-Albuquerque, Sterigenics (also a subsidiary of Sotera Health), and Steris] replenish their stocks. In an already supply-constrained market, sterilization by gamma radiation may not be the most attractive option for product conversion.
Considering the current challenges with EO sterilization, Table 4 outlines a number of actions that should be considered.
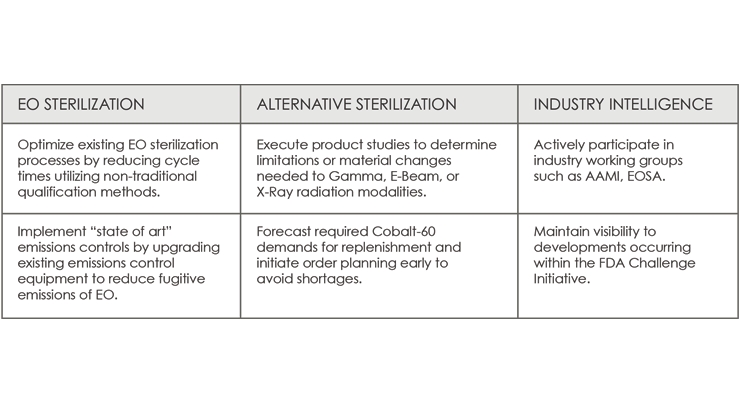
Table 4: Actions to consider regarding the current challenges surrounding EO sterilization.
Conclusion
The threat of an impending shortage of sterile medical devices is a direct and present risk to the industry and, more importantly, to patients. Announced Jan. 21, 2020, Medline Industries’ EO sterilization facility in Waukegan, Ill., is the most recent closure. Reportedly, other facilities nationwide are operating at approximately 90 percent capacity. Additional sterilization plant closures will push the elasticity of the supply chain to its limits. It’s a potentially serious crisis.
There are regulations for addressing potential supply chain disruptions in the drug and pharmaceutical space, but not for medical devices. All stakeholders, both public and private, must work together to find more effective means for monitoring, predicting, preventing, and mitigating potential future disruptions. It’s imperative today’s cautionary news guides our future work. As we all know, sterile medical devices save lives.
References
Over the past 18 years, Paul Boentges’ career has included quality leadership roles at Becton Dickinson, CareFusion, Devicor Medical, Ethicon Endo-Surgery (Johnson & Johnson), and Cardinal Health. His experience and expertise include sterilization sciences, quality management systems, and project management.
Sam Ramnarine, a Global Commodity Manager, has worked at Jabil for 19 years. His past roles have been in Quality, Supplier Quality, Jabil Defense and Aerospace, and Corporate Supply Chain for Jabil Healthcare. Prior to Jabil, Sam worked in plastics for 10 years in soft tool prototyping and OEM automotive supply chain.
For decades, EO has been successfully used as a terminal sterilization method for medical devices that cannot be adequately sterilized by other sterilization methods such as a radiation sterilization (gamma, e-beam, and X-ray), moist-heat sterilization (steam), dry-heat sterilization, vaporized hydrogen peroxide sterilization (VPA), and gas plasma sterilization (STERRAD).
Most major medical device manufacturers use EO as the primary method of terminal sterilization utilizing in-house capabilities or contract sterilization service providers such as Steris and Sterigenics. Approximately 50 percent of all medical devices are sterilized utilizing EO.
The Background
Ethylene oxide is one of 187 hazardous air pollutants or “air toxics” the EPA regulates under the Clean Air Act, placed initially on the federal list of carcinogens in 1985. In 2016, the EPA released a reassessment linking the chemical to diseases such as breast cancer, leukemia, and lymphomas. In addition to updating its risk value for ethylene oxide, the EPA has committed to working with state, local, and tribal air agencies to address this chemical.
In February 2019, the Sterigenics EO contract sterilization facility in Willowbrook, Ill., was ordered to cease operations as a result of air testing performed by the EPA’s Illinois branch office. Shortly thereafter, the Illinois legislature signed into law the nation’s strictest limits on the emission of the cancer-causing chemical (EO) as a result of an investigation into the relationship between emissions from the Sterigenics’ Willowbrook facility and the high rates of cancer in the surrounding area. As a result, Illinois legislation now mandates companies using EO must be completely contained within facilities and the results of annual testing must be submitted to the Illinois EPA. Consequently, Sterigenics has communicated it will no longer offer EO contract sterilization services at the Willowbrook facility.
Impact on Contract Sterilization Facility Volumes
According to the FDA’s Establishment Registration & Device Listing database, Sterigenics listed a total of 594 types of devices that underwent terminal EO sterilization in Willowbrook. These products included sutures, clamps, knives, stents, needles, and many other medical devices. It is estimated the Willowbrook facility could have represented as much as five percent of the company’s total EO volume.
As a result of the closure, medical device manufacturers have been aggressively working on identifying and qualifying alternate EO sterilization sites within the United States. Other states have initiated similar evaluations with sterilization sites that utilize EO as a sterilization agent. These sites include the Sterigenics EO contract sterilizer in Smyrna, Ga.; the Becton Dickinson in-house EO sterilizer in Covington, Ga.; the Viant Medical in-house EO sterilizer in Grand Rapids, Mich.; and the Medline EO in-house sterilizer in Mundelein, Ill.
FDA’s Shortages Response
Shutdowns of several EO sterilization sites have raised concerns about potential shortages in the healthcare industry since one half of all medical devices are sterilized using ethylene oxide. In response to the recent closure of several EO sterilization facilities over environmental concerns, the U.S. Food and Drug Administration (FDA) is taking aggressive steps to ensure hospitals, healthcare providers, and patients have access to medical devices that are safely and effectively sterilized.
Two federal agencies—the EPA and the FDA—have now both become collaborative stakeholders in managing this issue, but from different perspectives: reducing exposure to toxic emissions vs. ensuring critical medical device availability.
The FDA maintains a page on its website—Ethylene Oxide Sterilization Facility Updates1—with the sole purpose of providing timely information on any future actions the agency takes regarding medical device shortages and other activities associated with this issue. The following statement appears at the top of the page:
“The FDA is closely monitoring the supply chain effects of closures and potential closures of certain facilities that use ethylene oxide to sterilize medical devices prior to their use. The Agency is concerned about the future availability of sterile medical devices and the potential for medical device shortages that might impact patient care.”
FDA’s Innovation Challenges
On July 15, 2019, the FDA announced2 the launch of two public innovation challenges to encourage innovation in medical device sterilization—one focused on reducing emissions and the other encouraging alternative sterilization methods.
Challenge 1 is focused on identifying alternatives to EO sterilization methods. Table 1 lists the four participants selected from 24 applications.3

Table 1: The four participants selected from 24 applications to compete in the FDA’s challenge to identify alternatives to EO sterilization for medical devices.3
Challenge 2 is focused on developing strategies to reduce EO emissions. The FDA received 22 applications and selected eight participants (Table 2).4

Table 2: The eight participants selected from 22 applications to compete in the FDA’s challenge to develop strategies to reduce EO emissions.4
The FDA’s Center for Devices and Radiological Health encouraged participation in these challenges from organization such as medical devices companies, distributors, technology manufacturers, laboratories, academic institutions, healthcare facilities, professional societies, foundations, and not-for-profits. Based on current knowledge, medical device manufacturers (Johnson & Johnson, Becton Dickinson), sterilization equipment manufacturers, academic institutions, and professional societies (AAMI, AdvaMed) submitted responses to the FDA Challenge initiative.
Alternative Sterilization Method Feasibility
To proactively address potential product availability, as well as supply chain impacts, medical device manufacturers have launched internal product conversion initiatives to determine the feasibility of qualifying alternate sterilization methods for medical devices. Currently, radiation sterilization (gamma, e-beam, and X-ray) is the only feasible alternative due to its availability and long history of safe use within the healthcare industry. Unfortunately, many medical devices are not compatible with radiation sterilization due to product functionality limitations when exposed to radiation and its consequential degradative effects on materials (e.g., cross-linking, discoloration of plastics, embrittlement, etc.).
Of the three radiation sterilization modalities, gamma radiation utilizing cobalt-60 is the most prevalent. In North America, however, there is only one source for cobalt-60: Nordion, a subsidiary of Sotera Health. Demand for cobalt-60 in all radiation processing applications has been increasing in recent years (90 percent is used for the sterilization of disposable medical products, 5 percent for food irradiation, and 5 percent for other applications; Table 3) and will continue to ramp up over the next one to two years as more medical device manufacturers qualify products for terminal sterilization utilizing gamma radiation.

Table 3: Demand for cobalt-60 in all radiation processing applications has been increasing in recent years.
Another important factor to consider: since cobalt-60 decays continuously, supply of the isotope will be under additional pressure as existing sites currently providing gamma sterilization [e.g., Jabil-Albuquerque, Sterigenics (also a subsidiary of Sotera Health), and Steris] replenish their stocks. In an already supply-constrained market, sterilization by gamma radiation may not be the most attractive option for product conversion.
Considering the current challenges with EO sterilization, Table 4 outlines a number of actions that should be considered.

Table 4: Actions to consider regarding the current challenges surrounding EO sterilization.
Conclusion
The threat of an impending shortage of sterile medical devices is a direct and present risk to the industry and, more importantly, to patients. Announced Jan. 21, 2020, Medline Industries’ EO sterilization facility in Waukegan, Ill., is the most recent closure. Reportedly, other facilities nationwide are operating at approximately 90 percent capacity. Additional sterilization plant closures will push the elasticity of the supply chain to its limits. It’s a potentially serious crisis.
There are regulations for addressing potential supply chain disruptions in the drug and pharmaceutical space, but not for medical devices. All stakeholders, both public and private, must work together to find more effective means for monitoring, predicting, preventing, and mitigating potential future disruptions. It’s imperative today’s cautionary news guides our future work. As we all know, sterile medical devices save lives.
References
Over the past 18 years, Paul Boentges’ career has included quality leadership roles at Becton Dickinson, CareFusion, Devicor Medical, Ethicon Endo-Surgery (Johnson & Johnson), and Cardinal Health. His experience and expertise include sterilization sciences, quality management systems, and project management.
Sam Ramnarine, a Global Commodity Manager, has worked at Jabil for 19 years. His past roles have been in Quality, Supplier Quality, Jabil Defense and Aerospace, and Corporate Supply Chain for Jabil Healthcare. Prior to Jabil, Sam worked in plastics for 10 years in soft tool prototyping and OEM automotive supply chain.