Sam Brusco, Associate Editor03.04.20
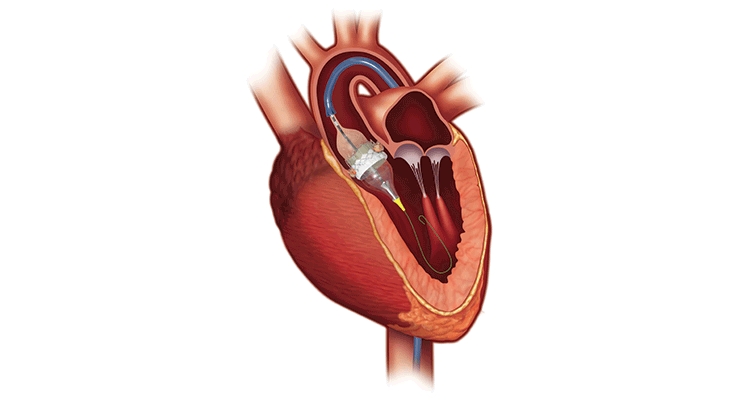
Developed by French interventional cardiologist Alain Cribier, M.D., the first catheter-based approach of balloon aortic valvuloplasty (BAV) took place in 1985 on a 77-year-old woman with inoperable severe aortic stenosis. Its main limitation was restenosis, which impacted most BAV patients within a year.
Inspired by a presentation of coronary artery stents, interventional cardiology trainee Henning Rud Andersen proposed a balloon-expandable valve could be similarly placed. So in 1992, he created a handmade metal stent and sutured on porcine aortic valves with a deflated balloon inside the valve. He then built a catheter delivery device inspired by the Cribier-Letac balloon catheter used in BAV procedures. Dr. Andersen’s hand-made valve measured an enormous 41-Fr.
He was unfortunately unable to find a company to further develop his approach, so he sold the patent he had been granted in 1995 for his invention to a startup called Percutaneous Valve Technologies (PVT), which was led by Dr. Cribier. PVT was greeted with great resistance on the part of the industry, with one investor famously calling transcatheter aortic valve implantation/replacement (TAVI/TAVR) “the most stupid project ever heard of.”
Dr. Cribier performed the first TAVR at the Charles Nicolle University Hospital in Rouen, France on a 57-year-old inoperable patient with critical calcific aortic stenosis. The patient had presented in cardiogenic shock with a dangerously low left ventricular ejection fraction of 12 percent and severe aortic stenosis. His multiple comorbidities of previous lung cancer, chronic pancreatitis, and leg ischemia made open-heart surgery a very high risk. BAV had failed the patient and he suffered from severe peripheral vascular disease.
After TAVR, the patient had remarkable hemodynamic improvement but died four months later due to non-procedure-related complications. Cribier, however, had confirmed the feasibility of TAVR via a transseptal approach, and it was quickly expanded to other patients. Edwards Lifesciences acquired PVT in 2004, leading to further evolution of delivery systems and approaches to TAVR. Since then, several structural heart procedures can be performed with a minimally-invasive, catheter-based approach.
“Transcatheter structural heart therapies include valve replacement, valve repair, LAA occlusion, cerebral protection, and defect closure,” said Maura Leahy, global marketing manager of TE Connectivity, a global industrial technology provider of connectivity and sensor solutions as well as minimally invasive delivery and access devices. “All these therapies have a common theme—success can only be achieved in the Cath Lab with accurate and precise access and delivery systems—and that is our focus. We first began commercial work in the structural heart field more than 17 years ago, working on steerable catheter shafts for valve deployment just months after the first TAVR case had been performed in Rouen, France. Since then, our solutions have enabled many advances in the minimally invasive structural heart field such as implant and balloon catheters, visualization systems, steerable delivery systems, and introducer sheaths.”
Last year saw efforts by both the U.S. Food and Drug Administration (FDA) and The Centers for Medicare & Medicaid Services (CMS) to expand access to TAVR procedures. Based on excellent trial results, last August the FDA expanded the indication for several TAVR systems to patients at low risk for death or major complications associated with open-heart surgery. The low-risk population was the final risk category to be approved for TAVR and experts widely expect the procedure to begin replacing a large swath of surgical valve replacement volume over the next few years.
“Last year we received approvals in the U.S. and Europe to treat patients suffering from severe aortic stenosis (AS) at low risk for open-heart surgery using Edwards SAPIEN 3 TAVR,” said Larry Wood, corporate VP, transcatheter aortic valve replacement at Edwards Lifesciences, an Irvine, Calif.-based manufacturer of technologies for structural heart disease and critical care monitoring. “This was a major milestone because, for the first time, all patients diagnosed with AS could be considered for TAVR based on factors such as anatomical considerations or other individual needs, rather than risk scores.”
Almost two months prior, CMS had also issued finalized national coverage determination for TAVR that reportedly offers more flexibilities for hospitals and providers. According to the policy, CMS will continue to cover TAVR under coverage with evidence development if the procedure is furnished in line with an FDA-approved indication. CMS also updated coverage criteria for hospitals and physicians to maintain a TAVR program (at least 50 aortic valve replacements and 300 percutaneous coronary interventions annually).
“An updated TAVR national coverage determination (NCD) by the U.S. Centers for Medicare and Medicaid Services also allowed for modernized requirements and a more streamlined patient evaluation process, meaningful enhancements that may help ensure equitable access for more patients suffering from severe AS,” added Wood.
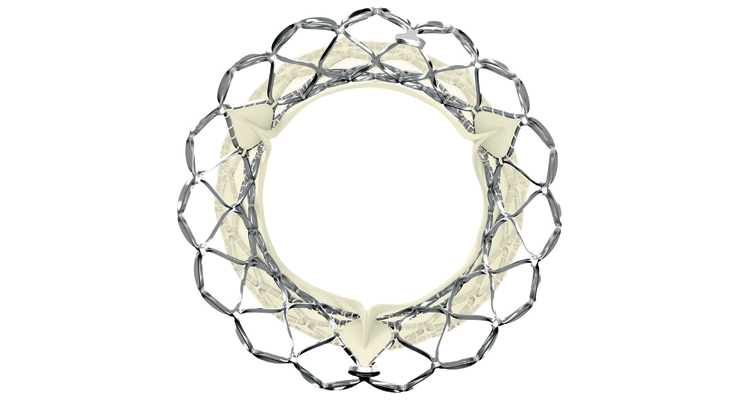
The SAPIEN 3 transcatheter heart valve (THV) system consists of a catheter-based artificial aortic heart valve, accompanying delivery system, and other implantation accessories. The valve is made of bovine pericardium and has a balloon-expandable, low-height cobalt-chromium frame with open cell geometry. SAPIEN 3’s polyethylene terephthalate skirt is also designed to minimize paravalvular leak.
“As published in The New England Journal of Medicine last year, the results of our randomized PARTNER 3 trial, which studied the SAPIEN 3 TAVR valve in low-risk patients, exceeded expectations by being superior to surgery,” said Wood. “These data demonstrated TAVR with the SAPIEN 3 valve gives low surgical risk patients with severe AS a treatment with better outcomes, less time in the hospital, and the ability to resume their everyday lives more quickly. This evidence helped lead to our FDA approval in August.”
According to trial data, the low-risk severe symptomatic aortic stenosis patients evaluated had three days of hospital stay with a TAVR procedure vs. seven days with open-heart surgery. Ninety-six percent of TAVR patients were discharged home from the hospital, while 73.1 percent of surgical patients were. And only 1.4 percent of TAVR patients had to be rehospitalized due to heart failure, against 3.6 percent who underwent surgery.
“An additional study examining quality of life in the PARTNER 3 patients, which was published in the Journal of the American College of Cardiology, demonstrated significant early and sustained advantages for low-risk patients treated with the SAPIEN 3 valve,” said Wood. “When the treatment strategies of TAVR and surgery were compared for low-risk patients, the TAVR patients improved more rapidly than surgery patients. This study showed, for the first time, patients treated with the SAPIEN 3 valve experienced a better quality of life even one year after the procedure.”
The company’s next step is evaluating severe AS patients who don’t show symptoms. In cardiology, this means no spontaneous symptoms are present: no angina, dizziness, lightheadedness or syncope during activity or spontaneously, and no extreme fatigue or any decrease in energy level. The problem is many of those symptoms are associated with normal aging, and many patients believe they are asymptomatic even if they have severe AS.
“Globally, diagnosis and treatment rates of AS remain low,” said Wood. “To address this, we continue to invest in groundbreaking trials and awareness initiatives. One of those trials, EARLY TAVR, is exploring the treatment of asymptomatic severe AS patients. We are asking the question: How can we prevent irreversible heart damage and other symptoms before they emerge? Initial evidence supports early aortic valve replacement, so we are continuing to enroll patients and study this.”
Global medical device manufacturer and MPO Top 30 leader Medtronic has been developing solutions to treat diseases affecting the structure of the heart since 1977, when the company introduced a revolutionary mechanical heart valve that had no welds, joints, or bends that could eventually weaken the structure of the valve. Since then, Medtronic’s structural heart business has expanded into heart valve repair, tissue valves, and the world’s first transcatheter valve in 2006.
“The Medtronic Melody TPV (transcatheter pulmonary valve) was the first transcatheter heart valve available anywhere in the world,” said a spokesperson for Medtronic. “It was originally approved under a Humanitarian Device Exemption (HDE), a regulatory approval for treatments intended for fewer than 4,000 U.S. patients per year. The Melody TPV has since received FDA pre-market approval based on strong clinical evidence from clinical studies demonstrating the valve’s effectiveness in delaying open-heart reoperation.”
Medtronic began its foray into TAVR in 2009, when the company acquired the CoreValve self-expanding TAVR system following its launch in Europe in 2007. The CoreValve system was the first-generation self-expandable valve introduced to the market. Although initial studies using the CoreValve system were positive, these first-generation TAVR devices were relatively bulky and used larger diameter catheters (18-Fr to 24-Fr), which raised the likelihood of procedural complications and relied heavily on operator expertise. Designs were eventually modified to include smaller profile delivery systems, but vascular complications, need for permanent pacing post-implant, paravalvular leak (PVL), and strokes were still risks.
The next-generation CoreValve Evolut R TAVR system featured a more secure seal against PVL, as well as a delivery system to make the valve recapturable and repositionable. The company launched its third-generation (Evolut PRO) TAVR system with recapturable and repositionable capabilities in 2016. The latest generation of Medtronic’s TAVR system—Evolut PRO+—launched in the U.S. last September. It’s designed to improve on the self-expanding Evolut platform and includes four valve sizes. According to Medtronic, Evolut PRO+ features the smallest delivery profile currently on the market.
“The four valve sizes are 23, 26, 29, and 34 mm with an external tissue wrap that increases surface contact area with the native anatomy for advanced sealing across the broadest range of patient anatomies,” said the Medtronic spokesperson. “A lower delivery profile for 23-29 mm valves for access down 5.0 mm access vessels with the 23-29 mm valves (6.0 mm for 34 mm) expands access to more patients and improves procedural safety for lower risk of vascular complication.”
Evolut PRO+’s self-expanding nitinol frame conforms to the native annulus with consistent radial force, resulting in enhanced valve sealing. Its external tissue wrap increases surface area contact with native anatomy and promotes tissue interaction meant to reduce incidences of PVL. For the first time, according to Medtronic, the extended tissue wrap has been added to the larger 34 mm valve size for patients with larger anatomies. Medtronic anticipates the system will help cardiac teams fine-tune their TAVR procedures.
“Several features and characteristics set the Evolut PRO+ TAVR System apart from other TAVR technologies,” Medtronic’s spokesperson said. “It features unmatched hemodynamic (bloodflow) performance versus SAVR, which is due to several factors. One factor is sustained large EOAs (effective orifice area) and low mean gradients over time—large, effective EOAs promote increased blood flow and minimize sizing mismatch, which helps patients maintain better bloodflow, enabling them to get back to everyday life. This is especially important for low-risk patients who may be slightly younger or more active than older AS patients too sick for surgical valve procedures.”
The FDA’s expanded TAVR indication for low-risk patients was also bestowed on Medtronic’s CoreValve, Evolut R, and Evolut PRO systems. The Evolut Low Risk Trial evaluated these three valve generations in over 1,400 patients, and data showed TAVR to have an excellent safety profile and be effective in low-risk patients.
“This is an exciting time for heart valve innovation, and in addition to bringing new technologies to the market such as Evolut PRO+, we are also committed to generating significant clinical and economic evidence for heart valve therapies,” said the Medtronic spokesperson. “This includes continued follow up in the Evolut Low Risk Trial, Low Risk Bicuspid, Apollo transcatheter mitral valve replacement (TMVR) trial, among others, including the Harmony TPV study (for congenital patients). We also have resources dedicated to R&D efforts for the next phase in TAVR innovation, which involves fine-tuning the system for even better deliverability, positioning accuracy, and visualization while looking at ways to improve coronary access and pacemaker rates.”
The transcatheter mitral valve repair/implantation (TMVR/TMVI) space is the next frontier now that TAVR has become a competitive market for the largest medical device makers with a structural heart portfolio. There are far fewer players in the TMVR market, but they’re mostly heavy hitters. Part of the reason is that accessing the mitral valve requires a different approach. TMVR isn’t just a quick “in and out”—to navigate around the aorta, the delivery catheter has to be large.
“Accessing areas of the heart such as the mitral valve can call for left heart access,” said Scott Larson, chief technology officer at TE Connectivity. “Managing the space constraint while ensuring accurate deployment is a clinical challenge and critical to a successful procedure. Visualization technologies can also assist with successful procedure outcomes.”
Abbott Laboratories’ MitraClip for mitral valve regurgitation—the first TMVR device on the market—was approved by the FDA in 2013. The firm also won CE mark approval at the end of January for its Tendyne TMVI system. Edwards’ Pascal TMVR received its CE mark last February. And last September, Medtronic won FDA approval to begin an early feasibility study for its Intrepid TMVR using the transfemoral approach. And Neovasc plans to begin a clinical feasibility study for its Tiara TMVR later this year.
Paris, France-based HighLife Medical is hot on their heels, however. The firm closed a 32 million Euro round to develop a TMVR system—its flagship product—last January. The proceeds were used to complete the development of HighLife’s transcatheter transseptal mitral valve implantation program, including trials in Europe to gain a CE mark and an early feasibility study in the U.S.
“We have completed the turnaround of our technology from a transapically delivered system to a fully percutaneous and transseptally delivered system,” said Georg Börtlein, HighLife Medical’s CEO and founder. “We have also completed our first-in-human clinical proof-of-concept phase and started a larger feasibility trial now running globally, in Australia, Europe, and the U.S.”
The HighLife Mitral Valve Replacement System consists of a valve prosthesis and a ring-shaped sub-annular implant (SAI). The valve self-centers and aligns itself upon delivery inside the SAI ring surrounding it. The system’s outer surface has atraumatic rounded edges with smooth symmetrical features. The valve can be implanted in native annuli with the largest axis starting at 35 mm, up to 50 mm.
“Our technology differs from competitive approaches by the fact that we create a dedicated landing zone for our valve as a first step that fully respects the patient’s hemodynamics,” said Börtlein. “Then, the operator can very easily deliver the valve over the wire very much like a TAVR with no need to drive orientation or axis. The valve self-aligns in the proper axis and leverages the native leaflets for good anchoring and sealing, avoiding any perivalvular leakage.”
Interventionists and patients prefer the transseptal route for TMVR because it avoids any open surgery. This route allows optimal exposure to address pathology in both the mitral and tricuspid valves, with the added advantage of extension up onto the dome of the left atrium if further exposure is needed. This exposure lets surgeons perform the full range of valve repair and replacement with minimal distortion of the heart.
“We want to complete our feasibility studies with the goal to establish solid clinical evidence of our product’s performance along with the confirmation that many users can safely carry out the procedure in a standardized way,” said Börtlein. “HighLife will then extend its clinical activity to pivotal studies in order to seek approval in all geographies.”
TAVR continues to be Edwards’ strongest market position, but the race to provide minimally invasive repair options for other heart valves in the U.S. continues. Abbott began a U.S. pivotal trial for its TriClip tricuspid valve repair system last September. Edwards beat Abbott to this market in Europe, securing a CE mark for its Cardioband tricuspid valve reconstruction system in April 2018.
“We are developing significant evidence to transform the care of patients living with mitral and tricuspid valve disease,” said Wood. “Currently, we are enrolling 10 clinical studies, including four pivotal trials. We know that many patients are living with diverse and complex structural heart diseases and are in need of better treatment options. Our TMTT (transcatheter mitral and tricuspid therapies) business is building a transcatheter-based portfolio to optimally treat this large and complex patient population.”
Catheter-based delivery systems play an integral role in advancing technologies used in transcatheter structural heart therapy. As the valves themselves get smaller, the catheter has also gotten smaller. Over time, catheter sizes used in transcatheter heart therapies have shrunk from 24-Fr to 14-Fr. The smaller the catheter size, the smaller the incision, ultimately resulting in less trauma to the patient.
And as transcatheter heart procedures continue to advance, physicians who specialize in minimally invasive procedures also have advancing expectations. They want improved performance and functionality in the catheters and delivery systems they use. One of the most crucial parameters is the ability to steer a catheter confidently and easily through challenging anatomies and deflect the tip for precise placement at the final target.
“Any successful design must start from the physician’s point of view,” said Larson. “Their preferences are firstly for low profile devices that cause minimum trauma to the patient. They also want delivery systems that enable smooth, accurate, and sometimes repositionable implant or repair device placement within the beating heart.”
“There are many and sometimes competing technical requirements that must be optimized to meet these clinical needs,” Larson continued. “Advanced multi-direction and multi-plane steering within the delivery system will assist with navigation and deployment, particularly for high load forces. Leveraging our expertise in metals, polymers, and hybrid technologies, we work with our customers to solve such complex challenges.”
Advancements in transcatheter heart procedures are also being driven by innovations in imaging technology. Better imaging makes an enormous difference in accurately placing a valve within the heart. Computed tomography (CT) initially used primarily for evaluating peripheral access has grown significantly. It’s now the gold standard for accurately sizing the annulus, determining the risk of annular imagery and coronary occlusion, and providing predictions in advance of the procedure. MRI is a more advanced visual modality than CT and could further improve visualization, but using it has many obstacles to overcome. Metallic braided or reinforced catheters can’t be used. MRI isn’t widely available in patient care areas, so patients would have to be moved mid-procedure.
However, procedure simulation in the cardiac catheterization lab can help physicians improve clinical skills within the CT imaging modality. Simulating a transcatheter cardiac procedure can decrease the learning curve for novices without compromising patient safety. Hands-on training using simulators also enhances the physician’s and paramedical staff’s motor skills and cognitive abilities in the interventional setting. Trainees can learn and practice the correct sequence of catheterization steps, enhancing visual and spatial thinking and improving hand-eye coordination.
It also bridges the gap in training diverse team members on new procedures and products, including myself and editor-in-chief Sean Fenske. At this year’s MD&M West expo in Anaheim, Calif., we respectively “operated” on a virtual patient in a simulated Cath Lab, “performing” a TAVR and stent implantation.
“We had a live simulator in our booth at this year’s MD&M West, where we recreated a typical Cath Lab environment,” said Leahy. “Booth visitors had an opportunity to perform a TAVR and other minimally invasive procedures using the same tools, workflow, and clinical considerations as happens in the Cath Lab with a real patient. For anyone seeking to deeply understand the challenges a physician faces in deploying a transcatheter valve, the best way to learn is hands-on. This first-hand experience brings a new dimension of insight for those designing devices for the structural heart therapies of the future.”
Inspired by a presentation of coronary artery stents, interventional cardiology trainee Henning Rud Andersen proposed a balloon-expandable valve could be similarly placed. So in 1992, he created a handmade metal stent and sutured on porcine aortic valves with a deflated balloon inside the valve. He then built a catheter delivery device inspired by the Cribier-Letac balloon catheter used in BAV procedures. Dr. Andersen’s hand-made valve measured an enormous 41-Fr.
He was unfortunately unable to find a company to further develop his approach, so he sold the patent he had been granted in 1995 for his invention to a startup called Percutaneous Valve Technologies (PVT), which was led by Dr. Cribier. PVT was greeted with great resistance on the part of the industry, with one investor famously calling transcatheter aortic valve implantation/replacement (TAVI/TAVR) “the most stupid project ever heard of.”
Dr. Cribier performed the first TAVR at the Charles Nicolle University Hospital in Rouen, France on a 57-year-old inoperable patient with critical calcific aortic stenosis. The patient had presented in cardiogenic shock with a dangerously low left ventricular ejection fraction of 12 percent and severe aortic stenosis. His multiple comorbidities of previous lung cancer, chronic pancreatitis, and leg ischemia made open-heart surgery a very high risk. BAV had failed the patient and he suffered from severe peripheral vascular disease.
After TAVR, the patient had remarkable hemodynamic improvement but died four months later due to non-procedure-related complications. Cribier, however, had confirmed the feasibility of TAVR via a transseptal approach, and it was quickly expanded to other patients. Edwards Lifesciences acquired PVT in 2004, leading to further evolution of delivery systems and approaches to TAVR. Since then, several structural heart procedures can be performed with a minimally-invasive, catheter-based approach.
“Transcatheter structural heart therapies include valve replacement, valve repair, LAA occlusion, cerebral protection, and defect closure,” said Maura Leahy, global marketing manager of TE Connectivity, a global industrial technology provider of connectivity and sensor solutions as well as minimally invasive delivery and access devices. “All these therapies have a common theme—success can only be achieved in the Cath Lab with accurate and precise access and delivery systems—and that is our focus. We first began commercial work in the structural heart field more than 17 years ago, working on steerable catheter shafts for valve deployment just months after the first TAVR case had been performed in Rouen, France. Since then, our solutions have enabled many advances in the minimally invasive structural heart field such as implant and balloon catheters, visualization systems, steerable delivery systems, and introducer sheaths.”
Last year saw efforts by both the U.S. Food and Drug Administration (FDA) and The Centers for Medicare & Medicaid Services (CMS) to expand access to TAVR procedures. Based on excellent trial results, last August the FDA expanded the indication for several TAVR systems to patients at low risk for death or major complications associated with open-heart surgery. The low-risk population was the final risk category to be approved for TAVR and experts widely expect the procedure to begin replacing a large swath of surgical valve replacement volume over the next few years.
“Last year we received approvals in the U.S. and Europe to treat patients suffering from severe aortic stenosis (AS) at low risk for open-heart surgery using Edwards SAPIEN 3 TAVR,” said Larry Wood, corporate VP, transcatheter aortic valve replacement at Edwards Lifesciences, an Irvine, Calif.-based manufacturer of technologies for structural heart disease and critical care monitoring. “This was a major milestone because, for the first time, all patients diagnosed with AS could be considered for TAVR based on factors such as anatomical considerations or other individual needs, rather than risk scores.”
Almost two months prior, CMS had also issued finalized national coverage determination for TAVR that reportedly offers more flexibilities for hospitals and providers. According to the policy, CMS will continue to cover TAVR under coverage with evidence development if the procedure is furnished in line with an FDA-approved indication. CMS also updated coverage criteria for hospitals and physicians to maintain a TAVR program (at least 50 aortic valve replacements and 300 percutaneous coronary interventions annually).
“An updated TAVR national coverage determination (NCD) by the U.S. Centers for Medicare and Medicaid Services also allowed for modernized requirements and a more streamlined patient evaluation process, meaningful enhancements that may help ensure equitable access for more patients suffering from severe AS,” added Wood.
The SAPIEN 3 transcatheter heart valve (THV) system consists of a catheter-based artificial aortic heart valve, accompanying delivery system, and other implantation accessories. The valve is made of bovine pericardium and has a balloon-expandable, low-height cobalt-chromium frame with open cell geometry. SAPIEN 3’s polyethylene terephthalate skirt is also designed to minimize paravalvular leak.
“As published in The New England Journal of Medicine last year, the results of our randomized PARTNER 3 trial, which studied the SAPIEN 3 TAVR valve in low-risk patients, exceeded expectations by being superior to surgery,” said Wood. “These data demonstrated TAVR with the SAPIEN 3 valve gives low surgical risk patients with severe AS a treatment with better outcomes, less time in the hospital, and the ability to resume their everyday lives more quickly. This evidence helped lead to our FDA approval in August.”
According to trial data, the low-risk severe symptomatic aortic stenosis patients evaluated had three days of hospital stay with a TAVR procedure vs. seven days with open-heart surgery. Ninety-six percent of TAVR patients were discharged home from the hospital, while 73.1 percent of surgical patients were. And only 1.4 percent of TAVR patients had to be rehospitalized due to heart failure, against 3.6 percent who underwent surgery.
“An additional study examining quality of life in the PARTNER 3 patients, which was published in the Journal of the American College of Cardiology, demonstrated significant early and sustained advantages for low-risk patients treated with the SAPIEN 3 valve,” said Wood. “When the treatment strategies of TAVR and surgery were compared for low-risk patients, the TAVR patients improved more rapidly than surgery patients. This study showed, for the first time, patients treated with the SAPIEN 3 valve experienced a better quality of life even one year after the procedure.”
The company’s next step is evaluating severe AS patients who don’t show symptoms. In cardiology, this means no spontaneous symptoms are present: no angina, dizziness, lightheadedness or syncope during activity or spontaneously, and no extreme fatigue or any decrease in energy level. The problem is many of those symptoms are associated with normal aging, and many patients believe they are asymptomatic even if they have severe AS.
“Globally, diagnosis and treatment rates of AS remain low,” said Wood. “To address this, we continue to invest in groundbreaking trials and awareness initiatives. One of those trials, EARLY TAVR, is exploring the treatment of asymptomatic severe AS patients. We are asking the question: How can we prevent irreversible heart damage and other symptoms before they emerge? Initial evidence supports early aortic valve replacement, so we are continuing to enroll patients and study this.”
Global medical device manufacturer and MPO Top 30 leader Medtronic has been developing solutions to treat diseases affecting the structure of the heart since 1977, when the company introduced a revolutionary mechanical heart valve that had no welds, joints, or bends that could eventually weaken the structure of the valve. Since then, Medtronic’s structural heart business has expanded into heart valve repair, tissue valves, and the world’s first transcatheter valve in 2006.
“The Medtronic Melody TPV (transcatheter pulmonary valve) was the first transcatheter heart valve available anywhere in the world,” said a spokesperson for Medtronic. “It was originally approved under a Humanitarian Device Exemption (HDE), a regulatory approval for treatments intended for fewer than 4,000 U.S. patients per year. The Melody TPV has since received FDA pre-market approval based on strong clinical evidence from clinical studies demonstrating the valve’s effectiveness in delaying open-heart reoperation.”
Medtronic began its foray into TAVR in 2009, when the company acquired the CoreValve self-expanding TAVR system following its launch in Europe in 2007. The CoreValve system was the first-generation self-expandable valve introduced to the market. Although initial studies using the CoreValve system were positive, these first-generation TAVR devices were relatively bulky and used larger diameter catheters (18-Fr to 24-Fr), which raised the likelihood of procedural complications and relied heavily on operator expertise. Designs were eventually modified to include smaller profile delivery systems, but vascular complications, need for permanent pacing post-implant, paravalvular leak (PVL), and strokes were still risks.
The next-generation CoreValve Evolut R TAVR system featured a more secure seal against PVL, as well as a delivery system to make the valve recapturable and repositionable. The company launched its third-generation (Evolut PRO) TAVR system with recapturable and repositionable capabilities in 2016. The latest generation of Medtronic’s TAVR system—Evolut PRO+—launched in the U.S. last September. It’s designed to improve on the self-expanding Evolut platform and includes four valve sizes. According to Medtronic, Evolut PRO+ features the smallest delivery profile currently on the market.
“The four valve sizes are 23, 26, 29, and 34 mm with an external tissue wrap that increases surface contact area with the native anatomy for advanced sealing across the broadest range of patient anatomies,” said the Medtronic spokesperson. “A lower delivery profile for 23-29 mm valves for access down 5.0 mm access vessels with the 23-29 mm valves (6.0 mm for 34 mm) expands access to more patients and improves procedural safety for lower risk of vascular complication.”
Evolut PRO+’s self-expanding nitinol frame conforms to the native annulus with consistent radial force, resulting in enhanced valve sealing. Its external tissue wrap increases surface area contact with native anatomy and promotes tissue interaction meant to reduce incidences of PVL. For the first time, according to Medtronic, the extended tissue wrap has been added to the larger 34 mm valve size for patients with larger anatomies. Medtronic anticipates the system will help cardiac teams fine-tune their TAVR procedures.
“Several features and characteristics set the Evolut PRO+ TAVR System apart from other TAVR technologies,” Medtronic’s spokesperson said. “It features unmatched hemodynamic (bloodflow) performance versus SAVR, which is due to several factors. One factor is sustained large EOAs (effective orifice area) and low mean gradients over time—large, effective EOAs promote increased blood flow and minimize sizing mismatch, which helps patients maintain better bloodflow, enabling them to get back to everyday life. This is especially important for low-risk patients who may be slightly younger or more active than older AS patients too sick for surgical valve procedures.”
The FDA’s expanded TAVR indication for low-risk patients was also bestowed on Medtronic’s CoreValve, Evolut R, and Evolut PRO systems. The Evolut Low Risk Trial evaluated these three valve generations in over 1,400 patients, and data showed TAVR to have an excellent safety profile and be effective in low-risk patients.
“This is an exciting time for heart valve innovation, and in addition to bringing new technologies to the market such as Evolut PRO+, we are also committed to generating significant clinical and economic evidence for heart valve therapies,” said the Medtronic spokesperson. “This includes continued follow up in the Evolut Low Risk Trial, Low Risk Bicuspid, Apollo transcatheter mitral valve replacement (TMVR) trial, among others, including the Harmony TPV study (for congenital patients). We also have resources dedicated to R&D efforts for the next phase in TAVR innovation, which involves fine-tuning the system for even better deliverability, positioning accuracy, and visualization while looking at ways to improve coronary access and pacemaker rates.”
The transcatheter mitral valve repair/implantation (TMVR/TMVI) space is the next frontier now that TAVR has become a competitive market for the largest medical device makers with a structural heart portfolio. There are far fewer players in the TMVR market, but they’re mostly heavy hitters. Part of the reason is that accessing the mitral valve requires a different approach. TMVR isn’t just a quick “in and out”—to navigate around the aorta, the delivery catheter has to be large.
“Accessing areas of the heart such as the mitral valve can call for left heart access,” said Scott Larson, chief technology officer at TE Connectivity. “Managing the space constraint while ensuring accurate deployment is a clinical challenge and critical to a successful procedure. Visualization technologies can also assist with successful procedure outcomes.”
Abbott Laboratories’ MitraClip for mitral valve regurgitation—the first TMVR device on the market—was approved by the FDA in 2013. The firm also won CE mark approval at the end of January for its Tendyne TMVI system. Edwards’ Pascal TMVR received its CE mark last February. And last September, Medtronic won FDA approval to begin an early feasibility study for its Intrepid TMVR using the transfemoral approach. And Neovasc plans to begin a clinical feasibility study for its Tiara TMVR later this year.
Paris, France-based HighLife Medical is hot on their heels, however. The firm closed a 32 million Euro round to develop a TMVR system—its flagship product—last January. The proceeds were used to complete the development of HighLife’s transcatheter transseptal mitral valve implantation program, including trials in Europe to gain a CE mark and an early feasibility study in the U.S.
“We have completed the turnaround of our technology from a transapically delivered system to a fully percutaneous and transseptally delivered system,” said Georg Börtlein, HighLife Medical’s CEO and founder. “We have also completed our first-in-human clinical proof-of-concept phase and started a larger feasibility trial now running globally, in Australia, Europe, and the U.S.”
The HighLife Mitral Valve Replacement System consists of a valve prosthesis and a ring-shaped sub-annular implant (SAI). The valve self-centers and aligns itself upon delivery inside the SAI ring surrounding it. The system’s outer surface has atraumatic rounded edges with smooth symmetrical features. The valve can be implanted in native annuli with the largest axis starting at 35 mm, up to 50 mm.
“Our technology differs from competitive approaches by the fact that we create a dedicated landing zone for our valve as a first step that fully respects the patient’s hemodynamics,” said Börtlein. “Then, the operator can very easily deliver the valve over the wire very much like a TAVR with no need to drive orientation or axis. The valve self-aligns in the proper axis and leverages the native leaflets for good anchoring and sealing, avoiding any perivalvular leakage.”
Interventionists and patients prefer the transseptal route for TMVR because it avoids any open surgery. This route allows optimal exposure to address pathology in both the mitral and tricuspid valves, with the added advantage of extension up onto the dome of the left atrium if further exposure is needed. This exposure lets surgeons perform the full range of valve repair and replacement with minimal distortion of the heart.
“We want to complete our feasibility studies with the goal to establish solid clinical evidence of our product’s performance along with the confirmation that many users can safely carry out the procedure in a standardized way,” said Börtlein. “HighLife will then extend its clinical activity to pivotal studies in order to seek approval in all geographies.”
TAVR continues to be Edwards’ strongest market position, but the race to provide minimally invasive repair options for other heart valves in the U.S. continues. Abbott began a U.S. pivotal trial for its TriClip tricuspid valve repair system last September. Edwards beat Abbott to this market in Europe, securing a CE mark for its Cardioband tricuspid valve reconstruction system in April 2018.
“We are developing significant evidence to transform the care of patients living with mitral and tricuspid valve disease,” said Wood. “Currently, we are enrolling 10 clinical studies, including four pivotal trials. We know that many patients are living with diverse and complex structural heart diseases and are in need of better treatment options. Our TMTT (transcatheter mitral and tricuspid therapies) business is building a transcatheter-based portfolio to optimally treat this large and complex patient population.”
Catheter-based delivery systems play an integral role in advancing technologies used in transcatheter structural heart therapy. As the valves themselves get smaller, the catheter has also gotten smaller. Over time, catheter sizes used in transcatheter heart therapies have shrunk from 24-Fr to 14-Fr. The smaller the catheter size, the smaller the incision, ultimately resulting in less trauma to the patient.
And as transcatheter heart procedures continue to advance, physicians who specialize in minimally invasive procedures also have advancing expectations. They want improved performance and functionality in the catheters and delivery systems they use. One of the most crucial parameters is the ability to steer a catheter confidently and easily through challenging anatomies and deflect the tip for precise placement at the final target.
“Any successful design must start from the physician’s point of view,” said Larson. “Their preferences are firstly for low profile devices that cause minimum trauma to the patient. They also want delivery systems that enable smooth, accurate, and sometimes repositionable implant or repair device placement within the beating heart.”
“There are many and sometimes competing technical requirements that must be optimized to meet these clinical needs,” Larson continued. “Advanced multi-direction and multi-plane steering within the delivery system will assist with navigation and deployment, particularly for high load forces. Leveraging our expertise in metals, polymers, and hybrid technologies, we work with our customers to solve such complex challenges.”
Advancements in transcatheter heart procedures are also being driven by innovations in imaging technology. Better imaging makes an enormous difference in accurately placing a valve within the heart. Computed tomography (CT) initially used primarily for evaluating peripheral access has grown significantly. It’s now the gold standard for accurately sizing the annulus, determining the risk of annular imagery and coronary occlusion, and providing predictions in advance of the procedure. MRI is a more advanced visual modality than CT and could further improve visualization, but using it has many obstacles to overcome. Metallic braided or reinforced catheters can’t be used. MRI isn’t widely available in patient care areas, so patients would have to be moved mid-procedure.
However, procedure simulation in the cardiac catheterization lab can help physicians improve clinical skills within the CT imaging modality. Simulating a transcatheter cardiac procedure can decrease the learning curve for novices without compromising patient safety. Hands-on training using simulators also enhances the physician’s and paramedical staff’s motor skills and cognitive abilities in the interventional setting. Trainees can learn and practice the correct sequence of catheterization steps, enhancing visual and spatial thinking and improving hand-eye coordination.
It also bridges the gap in training diverse team members on new procedures and products, including myself and editor-in-chief Sean Fenske. At this year’s MD&M West expo in Anaheim, Calif., we respectively “operated” on a virtual patient in a simulated Cath Lab, “performing” a TAVR and stent implantation.
“We had a live simulator in our booth at this year’s MD&M West, where we recreated a typical Cath Lab environment,” said Leahy. “Booth visitors had an opportunity to perform a TAVR and other minimally invasive procedures using the same tools, workflow, and clinical considerations as happens in the Cath Lab with a real patient. For anyone seeking to deeply understand the challenges a physician faces in deploying a transcatheter valve, the best way to learn is hands-on. This first-hand experience brings a new dimension of insight for those designing devices for the structural heart therapies of the future.”