Seth Goldenberg, Ph.D., Vice President, Vault Medical Device & Diagnostics, Veeva Systems11.04.19
Market access is a significant challenge for today’s medical device and diagnostics manufacturers. New global regulatory requirements are one contributing factor. For example, Europe’s new Medical Device Regulation (MDR) will require companies to provide stronger clinical evidence on the efficacy, safety, and value of their products. In the U.S., the Food and Drug Administration (FDA) set new expectations for risk assessment for medical devices and diagnostics.
In addition to more complicated regulations, the industry now faces demands from a larger group of diverse stakeholders—regulators, payers, providers, hospitals, hospital networks, patients, and advocacy groups—each with different priorities and varying levels of influence on market access. Device and diagnostics companies must now provide broader, more value-based data to obtain coverage from payers. This information will also satisfy inquiries from budget-conscious public healthcare systems looking more holistically at the long-term benefits on patient care.
Real-world evidence (RWE) is becoming increasingly important for manufacturers to demonstrate value beyond efficacy and safety standards. However, the scale and complexity of unstructured data makes it difficult for companies to assemble and analyze it because much of this data is spread across information silos. To unlock market access, manufacturers require new data-driven solutions that can bring together diverse sources of information for a more complete view of all the ways their products can help patients.
Addressing the Concerns of More Diverse Stakeholders
Over the past three decades, device companies only needed strong clinical evidence a product worked as described and provided a clinical benefit to patients.1 In addition to product safety and efficacy evidence, companies must now show health economic information that demonstrates product value to a wide range of stakeholders—each of which may define value differently.
National hospital networks, for example, often buy devices in bulk and want to know the value of using new products or procedures before making a large investment. Medical device and diagnostics manufacturers must provide meaningful information beyond statistical outcome, such as findings on the long-term benefits to the healthcare system and patient. In addition to a product’s intended purpose, manufacturers must outline product value, long-term cost savings, length of patient recovery, and other information to help healthcare decision-makers determine the products that will provide the best patient care within their budgets.
The same is true for payers, which typically have the greatest influence on market access. In the past, a CPT code plus coverage meant reimbursement. However, a CPT code no longer equates to automatic reimbursement from insurers. Today, medical device and diagnostics companies must show proof of long-term value to stakeholders. They must present clinical data and real-world evidence their product will save money because of a shortened hospital stay, elimination of a secondary procedure, or other benefits.
“Insurance providers, both private and public, are no longer relying solely on the opinions of medical experts or clinical trial data to make coverage decisions. They are increasingly looking at cost effectiveness as the gating factor for payment,” explained Shantanu Gaur, M.D., co-founder and chief scientific officer at medical device company Allurion Technologies.2
Leveraging All the Right Data to Demonstrate Value
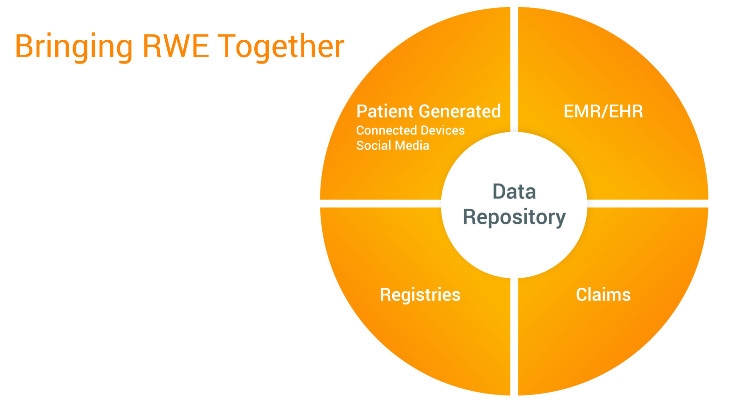
There are many databases medical device and diagnostics companies can mine to find evidence of their product’s value to patients, but no single source provides a complete view. Most common examples are claims databases, EMR/EHRs, health surveys, social media, patient-generated health data (PGHD), and patient registries.
Claims databases combine all patient claims submitted by healthcare providers (HCPs) to payers. They are a common source of information for payers, but don’t include any other information. HCPs input routine clinical and laboratory data into EMR/EHR systems, but some worry about the quality of the data in these systems as physicians experience “EMR fatigue.”
PGHD offers data from patients, family members, and caregivers. It usually includes health history, biometric data, symptoms, and lifestyle choices. PGHD can provide an inside view of a patient’s health, quality of life, and adherence to a treatment plan, enabling timely intervention before a costly care episode. However, this data source also comes with complications. For example, providers need to set up advanced analytical tools, build processes into their workflow, and determine what information to include in patient EHRs.
Not all providers can take these extra measures. Some providers are concerned about potential negative impacts on efficiency, increased liability risk, and setting realistic patient expectations. Some patients’ low health and technical literacy could also negatively affect the accuracy of PGHD. Other obstacles include the need to manage potential security risks, address patients’ privacy concerns, and standardize the data collected from multiple devices.
In addition to these sources, there are open data sources such as pharmacy databases and health insurance records. These systems are primarily maintained for billing and administration, but can also be used by researchers, insurers, health authorities, and other stakeholders to provide long-term data on the impact of health interventions on the healthcare system in real-world observational studies.
Payers, governments, and hospital networks are already leveraging various combinations of these data sources to determine market access. Likewise, medical device and diagnostics companies must look at it all, including more RWE to identify trends and fill in gaps from any individual data sources. By combining all sources, manufacturers can provide a holistic analysis of a product’s long-term value to regulators, healthcare organizations, providers, and more importantly, patients. Unfortunately, this has been a challenge for medical device and diagnostics manufacturers.
Aggregating Various Sources of Real-World Evidence
RWE has not lived up to expectations, largely due to a lack of technology available to manage it all effectively. To add complexity, real-world data comes in both structured and unstructured formats, making it difficult to bring it all together for a complete picture. Device/diagnostics companies can only look at one data set at a time, rather than in aggregate for greater analysis.
Fortunately, technology is now available to bring structured and unstructured data together at any scale as a “data lake” in a single, centralized system. Medical device and diagnostics companies can store this data without having to structure it first, and can then apply next-generation analytics tools—from dashboards and visualizations to big-data processing, real-time analytics, and artificial intelligence—to identify trends that reinforce a strong product value proposition.
Not too long ago, medical device and diagnostics manufacturers didn’t require such sophisticated systems, and instead could get by using manual tools to analyze fewer data points—they only needed to appeal to a limited audience with fewer demands. A published paper was often sufficient evidence to prove the viability of a product or procedure. With greater data demands from a wider range of stakeholders and much larger studies across regions, these methods are no longer sufficient. Today, advanced clinical data management technology integrated with electronic data capture functionality enables manufacturers to manage all of their data in one system.
Medical device and diagnostics companies also now require flexible solutions to handle changing requirements and more sources of relevant data. Compared with the rigidity of FDA requirements, payer requests for data are fuzzier, often changing cyclically and differing among private insurers. Agile, cloud-based systems that are easily adaptable to changing needs are an important foundation for communicating product value to secure faster market access. Additionally, these systems should include data functionality robust enough to manage large data lakes and enable advanced data analysis.
Modernize to Get Access
As regulators, payers, and healthcare systems restructure market access for the medical device and diagnostics industry, manufacturers require modern, flexible technology to analyze both unstructured and structured data from multiple data sources in one environment. This is the key to unlocking those hard-to-budge market access doors. New, fuller data sets and sophisticated technologies will empower device and diagnostics companies to bring their innovative therapies to patients fast.
References
Seth Goldenberg, a former director at NAMSA where he supported medical device companies from inception through commercialization and post-market activities, understands this new paradigm better than most. Now, as vice president of Medical Devices & Diagnostics at Veeva Systems, Goldenberg is overseeing the development of a sophisticated cloud technology platform that will drive the process efficiencies and speed that the industry needs today. New to Veeva, Goldenberg is excited to bring his passion in medical devices to the life sciences cloud technology company. He can be reached at seth.goldenberg@veeva.com.
In addition to more complicated regulations, the industry now faces demands from a larger group of diverse stakeholders—regulators, payers, providers, hospitals, hospital networks, patients, and advocacy groups—each with different priorities and varying levels of influence on market access. Device and diagnostics companies must now provide broader, more value-based data to obtain coverage from payers. This information will also satisfy inquiries from budget-conscious public healthcare systems looking more holistically at the long-term benefits on patient care.
Real-world evidence (RWE) is becoming increasingly important for manufacturers to demonstrate value beyond efficacy and safety standards. However, the scale and complexity of unstructured data makes it difficult for companies to assemble and analyze it because much of this data is spread across information silos. To unlock market access, manufacturers require new data-driven solutions that can bring together diverse sources of information for a more complete view of all the ways their products can help patients.
Addressing the Concerns of More Diverse Stakeholders
Over the past three decades, device companies only needed strong clinical evidence a product worked as described and provided a clinical benefit to patients.1 In addition to product safety and efficacy evidence, companies must now show health economic information that demonstrates product value to a wide range of stakeholders—each of which may define value differently.
National hospital networks, for example, often buy devices in bulk and want to know the value of using new products or procedures before making a large investment. Medical device and diagnostics manufacturers must provide meaningful information beyond statistical outcome, such as findings on the long-term benefits to the healthcare system and patient. In addition to a product’s intended purpose, manufacturers must outline product value, long-term cost savings, length of patient recovery, and other information to help healthcare decision-makers determine the products that will provide the best patient care within their budgets.
The same is true for payers, which typically have the greatest influence on market access. In the past, a CPT code plus coverage meant reimbursement. However, a CPT code no longer equates to automatic reimbursement from insurers. Today, medical device and diagnostics companies must show proof of long-term value to stakeholders. They must present clinical data and real-world evidence their product will save money because of a shortened hospital stay, elimination of a secondary procedure, or other benefits.
“Insurance providers, both private and public, are no longer relying solely on the opinions of medical experts or clinical trial data to make coverage decisions. They are increasingly looking at cost effectiveness as the gating factor for payment,” explained Shantanu Gaur, M.D., co-founder and chief scientific officer at medical device company Allurion Technologies.2
Leveraging All the Right Data to Demonstrate Value
There are many databases medical device and diagnostics companies can mine to find evidence of their product’s value to patients, but no single source provides a complete view. Most common examples are claims databases, EMR/EHRs, health surveys, social media, patient-generated health data (PGHD), and patient registries.
Claims databases combine all patient claims submitted by healthcare providers (HCPs) to payers. They are a common source of information for payers, but don’t include any other information. HCPs input routine clinical and laboratory data into EMR/EHR systems, but some worry about the quality of the data in these systems as physicians experience “EMR fatigue.”
PGHD offers data from patients, family members, and caregivers. It usually includes health history, biometric data, symptoms, and lifestyle choices. PGHD can provide an inside view of a patient’s health, quality of life, and adherence to a treatment plan, enabling timely intervention before a costly care episode. However, this data source also comes with complications. For example, providers need to set up advanced analytical tools, build processes into their workflow, and determine what information to include in patient EHRs.
Not all providers can take these extra measures. Some providers are concerned about potential negative impacts on efficiency, increased liability risk, and setting realistic patient expectations. Some patients’ low health and technical literacy could also negatively affect the accuracy of PGHD. Other obstacles include the need to manage potential security risks, address patients’ privacy concerns, and standardize the data collected from multiple devices.
In addition to these sources, there are open data sources such as pharmacy databases and health insurance records. These systems are primarily maintained for billing and administration, but can also be used by researchers, insurers, health authorities, and other stakeholders to provide long-term data on the impact of health interventions on the healthcare system in real-world observational studies.
Payers, governments, and hospital networks are already leveraging various combinations of these data sources to determine market access. Likewise, medical device and diagnostics companies must look at it all, including more RWE to identify trends and fill in gaps from any individual data sources. By combining all sources, manufacturers can provide a holistic analysis of a product’s long-term value to regulators, healthcare organizations, providers, and more importantly, patients. Unfortunately, this has been a challenge for medical device and diagnostics manufacturers.
Aggregating Various Sources of Real-World Evidence
RWE has not lived up to expectations, largely due to a lack of technology available to manage it all effectively. To add complexity, real-world data comes in both structured and unstructured formats, making it difficult to bring it all together for a complete picture. Device/diagnostics companies can only look at one data set at a time, rather than in aggregate for greater analysis.
Fortunately, technology is now available to bring structured and unstructured data together at any scale as a “data lake” in a single, centralized system. Medical device and diagnostics companies can store this data without having to structure it first, and can then apply next-generation analytics tools—from dashboards and visualizations to big-data processing, real-time analytics, and artificial intelligence—to identify trends that reinforce a strong product value proposition.
Not too long ago, medical device and diagnostics manufacturers didn’t require such sophisticated systems, and instead could get by using manual tools to analyze fewer data points—they only needed to appeal to a limited audience with fewer demands. A published paper was often sufficient evidence to prove the viability of a product or procedure. With greater data demands from a wider range of stakeholders and much larger studies across regions, these methods are no longer sufficient. Today, advanced clinical data management technology integrated with electronic data capture functionality enables manufacturers to manage all of their data in one system.
Medical device and diagnostics companies also now require flexible solutions to handle changing requirements and more sources of relevant data. Compared with the rigidity of FDA requirements, payer requests for data are fuzzier, often changing cyclically and differing among private insurers. Agile, cloud-based systems that are easily adaptable to changing needs are an important foundation for communicating product value to secure faster market access. Additionally, these systems should include data functionality robust enough to manage large data lakes and enable advanced data analysis.
Modernize to Get Access
As regulators, payers, and healthcare systems restructure market access for the medical device and diagnostics industry, manufacturers require modern, flexible technology to analyze both unstructured and structured data from multiple data sources in one environment. This is the key to unlocking those hard-to-budge market access doors. New, fuller data sets and sophisticated technologies will empower device and diagnostics companies to bring their innovative therapies to patients fast.
References
- STAT News, “3 Hurdles to Bringing Medical Devices to Market,” by Shantanu Gaur, (September 27, 2018).
- Med Device Online, “Getting Medtech Covered: Payer Strategy Best Practices” by Tong, Kuo and Navigant, Raj Stewart, (2018).
Seth Goldenberg, a former director at NAMSA where he supported medical device companies from inception through commercialization and post-market activities, understands this new paradigm better than most. Now, as vice president of Medical Devices & Diagnostics at Veeva Systems, Goldenberg is overseeing the development of a sophisticated cloud technology platform that will drive the process efficiencies and speed that the industry needs today. New to Veeva, Goldenberg is excited to bring his passion in medical devices to the life sciences cloud technology company. He can be reached at seth.goldenberg@veeva.com.