Michael Barbella, Managing Editor10.09.19
Smaller, faster, cheaper.
Those three words have become the unofficial motto of medtech development in recent decades as clinicians and innovators worked to improve both patient care and access. Driven by the advent of digital health and minimally invasive technologies, the slogan has spawned numerous inventions that are less intrusive and better suited for home use (think oxygen concentrators, CPAP machines, cochlear implants, and pacemakers).
“Medical manufacturers are moving entire systems—from home-based and clinical devices to imaging applications—into a portable unit the size of a cell phone or smaller,” states a 2016 whitepaper from Microsemi Corporation, an Aliso Viejo, Calif.-based provider of semiconductor and system solutions for the aerospace and defense, communications, data center, and industrial markets. “What was once huge equipment tethered to a wall has become available in mobile clinics, ambulances, and in a doctor’s bag for house calls.”
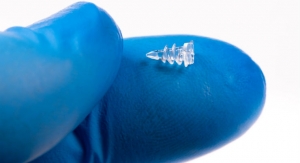
Some devices are now even small enough to be lost inside the doctor’s bag. Pacemakers, for example, have shrunk from the size of a cathode ray tube television set to that of a large pill. Medtronic plc spent a decade developing the one-inch Micra, a device small enough to be inserted through a groin vein and fed through the femoral artery to the heart. At one-tenth the size of traditional pacemakers, Micra is an upshot of Medtronic’s “deep miniaturization” program, an initiative that aims to shrink medical devices by up to 90 percent.
“We will no longer pace the heart in the way we have in the last 20 to 30 years,” John Hummel, M.D., a cardiologist who participated in Micra clinical trials, said when the device received U.S. Food and Drug Administration approval in April 2016. “This is fundamentally a paradigm shift in how we’ll deliver this therapy. We are looking at the beginning of the future.”
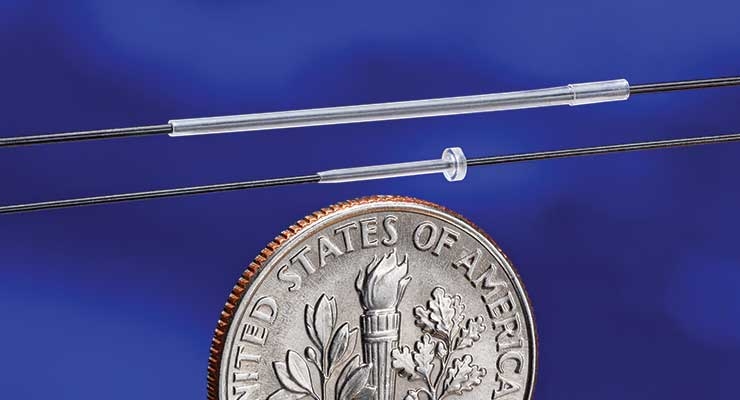
The key to ensuring that future is micromolding, a highly specialized manufacturing process used to create tiny, 3D components with small features and dimensional tolerances. Micromolded parts are made from various thermoplastic materials including PEEK (polyether ether ketone), liquid crystal polymer, and nylon, though components also can be comprised of optical and medical-grade materials. The types of substances used allow for mold sizes to be smaller than that of a dime.
To better understand the factors and trends driving the micromolding industry, Medical Product Outsourcing spoke with numerous industry professionals over the last month. They included:
Michael Barbella: What are the latest advancements in micromolding technologies?
Lars Gerding: We see more and more standardization around the demolding process and with inline quality inspection/vision control.
Patrick Haney: As of late, MTD has been taking a serious stance on understanding and manipulating our final products on a molecular level. With our constant effort to learn, we have launched a series of characterization projects to analyze the morphology of our molded components. Equipped with this knowledge, we are beginning to develop custom and original technology to incorporate into our injection molds to manipulate such things.
Scott Herbert: Significant advancements in control of materials.
Aaron Johnson: Like a lot of manufacturing services, micro molding innovation is driven by the demand of our customers pushing the limits of their own technology. In a lot of cases designs are asking to do more in the same space, or commonly, more in less. This demand puts a lot of pressure on the supply chain to constantly be looking for solutions. Advancements like extreme thin-wall molding, 2-shot micro molding, and automated insert molding are a direct result from the marketplace.
Ben Passetti: Injection molding equipment and technology in general. New micromolding sensors have come a long way and have been made specifically to fit the molds (the sensors used to be too big).
Rob Schwenker: Advancements in micromolding for Spectrum Plastics continue to be focused on automated part handling and measurement systems. The ability to mold parts is only as good as your ability to prove that your precision is accurate. It is also important to build measurement systems early on during the development phase as it is during validation.
Wayne Shakal: Tooling: The advancement of 5-axis micro CNC machine tools and micro sinker EDM allowing for micron level detail and accuracy. Micromolding: New style injection units that enable molders to inject with sub-1 gram shot sizes with minimal degradation and very high shot-to-shot accuracy. The advancement in the miniaturization of pressure and temperature sensors is allowing the ability to better control processes and provide real-time monitoring and in some cases closed-loop process control. Validation: The advancement of in-house CT scanning and 3D laser scanning technologies have given us the ability to achieve passing gauge R&R’s to inspect plastic dimensions with micron tolerances.
Peter Wojtas: Runnerless or reduced flow path molds to conserve expensive materials will lead to machine manufacturers redesigning to achieve high precision with extremely small shot sizes. Other recent advancements include custom screw designs for exotic materials, improved reduced-wall-thickness-filling abilities, in-mold annealing and stress relief, and improved material and mold monitoring systems (smaller mold sensors, real-time moisture data).
Barbella: What challenges are associated with producing small, delicate, intricate micromolded components for medical devices? How does your company meet those challenges?
Jared Cicio: Handling and packaging can be challenging with small, delicate parts. Bulk packing micro parts can be the easiest method, but with small, intricate, sharp features, figuring out a way to get the parts from the molding machine to the customer can prove to be just as difficult as producing the part. More micro parts require individually packing in custom packaging or trays to protect the features and allow for easier handling. There are many other challenges that come with producing such small parts. Testing is performed to determine the safest temperature to keep machines at while material is in the barrel, but not running. We also have ionizing fans in place to avoid any static which can lead to airborne contamination attaching to molded parts. We also incorporate multiple covered drop zones that catch our parts and keep them safe from contamination until they are packaged.
Gerding: Standardization, dimensional control, in-line close loop feedback, and reproducibility with no rework required. In addition, part handling (demolding, bagging, etc.) of micro-sized silicone products is a challenge in itself.
Haney: The primary challenges involved with producing such small components can be summarized as “the need for absolute precision and attention to detail in every category imaginable.” When it comes to producing components that are intended to serve such a critical application, we need to make sure that we are controlling every aspect of the manufacturing process. This means everything from unprecedented precision in machining the molding components, to monitoring and controlling the final morphology of the molded product. Our top down facility allows us to achieve such feats by allowing MTD to keep every aspect of the product line in-house, from mold making, to processing, to inspection, and material characterization.
Herbert: The challenges are plentiful, they are all different and unique. Most are lumped in to just a few areas. Part handling, part orientation, and in some cases static, all of which are controlled by custom automation and advancements in static control.
Johnson: One of the biggest challenges in producing micro components for medical devices is making sure you have the systems in place for quality control, especially when dealing with tight tolerance plastic components. Building micro components with micron tolerances is difficult enough. The validation process for most medical device components is very demanding. At Accumold, we follow the ISO 13485 quality management process throughout the organization to ensure the product that we make meets the customer’s expectations. We also work very closely with our customers to understand their exact needs from the very beginning.
Passetti: Mold design and construction are critical. Achieving shot-to-shot consistency is one of the largest challenges that we face and this applies more to the processing/handling side of things.
However, it all depends on the machine and the pellets that you use along with the compression. These things determine the performance the most. We partner with some of the best tool shops in the world to overcome these challenges. We also have the capability in house to maintain the molds and a high level of tooling. We select some of the best machines in the world and test them rigorously, and modify them based on need and improved repeatability. The age of assets is less than five years old to keep relevant with technology—this improves efficiencies as time goes on.
Schwenker: Some of the most demanding challenges surround micromolding over inserts, the placement and management of inserts requires precise placement, or you risk tool damage. In many cases automation is not implemented until products have reached a certain volume threshold, so operators who are skilled at working under microscopes with calm controlled movements are highly valued.
Training programs around working under magnification and understanding operator’s skill sets in these areas can often be the reason for the success of a program. These same challenges apply to managing the quality and inspection of parts as well.
Shakal: Tooling: Isometric has invested in state-of-the-art micro machining and mold technology that allows us to produce part geometries that would be deemed impossible by most mold builders and molders. Micromolding: Coupled with our vast amount of micro-focused research and development, we’ve established various tooling and molding methods that give us additional fill capabilities. It almost always comes down to the ability to fill and the difference between failure and success is often less than .0001 grams (0.1 mg) of fill.
Barbella: What factors are typically taken into consideration when selecting materials for micromolded device components?
Donna Bibber: Materials are typically selected for their function first. Flexibility, rigidity, and strength.
The classification of device and whether there has been a predicate device using the actual grade of material. Also, is the material able to be manufactured/molded to the size, wall thickness ranges (melt flow rate, molecular weight distribution, fillers preventing flow, feature size replication)? Is the material lot-to-lot variation or cavity-to-cavity variation able to be extrapolated in scale to be within the acceptable Cpk range?
Gerding: The materials used in silicone micromolding are the same materials used in larger parts. The key to success lies in how the materials are handled and dispensed. You may think viscosity would play a role in order to fill small features along the melt flow path but silicone flows like water. Flash control is much more important in micromolding, as flash can be larger than the micro part.
Haney: There are many factors to consider during the material selection phase of a project. Through the manufacturing process all the way to application, each phase of a micromolded project presents its own set of unique aspects to consider. First and foremost, items like mechanical, thermal, and degradation mechanisms all must meet the requirements of the intended application. However, in addition to the standard “form, fit, and function” considerations, properties such a crystallinity, shear sensitivity, compressibility, and other rheological aspects become exponentially more important when scaling down to a micro-sized component. We must also make sure the material selected will conform to such requirements in order to manufacture a reliable product.
Herbert: Material selection is very critical and application-specific. Implants and resorbable materials are all dependent on the end use.
Johnson: It’s common to start the material selection process by looking at the inherent properties of the material itself. You might be looking for biocompatibility, heat resistance or other attributes the material might provide. The challenge comes when trying to match the material selection with the desired geometries. With micromolding the smaller the feature you’re looking to produce, the more material selection becomes a critical part of the process. For example, PEEK is a common material selected in the medical device industry but if you’ve ever worked with this particular resin it isn’t always friendly to micro features. Understanding the tool build and the processing side of micromolding becomes very important in the design for manufacturability. In these cases, choosing a molder with experience understanding resins and micro feature performance will be an advantage.
Passetti: Pellet size and consistency of the raw material are the two main factors that are taken into consideration when selecting materials for micromolded device components.
Schwenker: It is important to understand the rheology of the material and minimize material degradation. Because we are dealing with such small volumes of material, process techniques can have a significant impact on part integrity.
Barbella: What material advances are currently impacting micromolding capabilities?
Bibber: Particle size distribution (pellet size), and pre-compounding effectiveness, especially if specialty pantones are required.
Material melt flow ranges to be specified at purchase to address micron tolerance Cpk.
New materials used in prototyping methods (3D printing) can show false negatives that a product will not be reproducible. Material selection and scalability at the micro level requires using materials, processes, and manufacturing methods that are constant from prototype to pilot to production so learnings and test results do not require repeating. Assumptions made at prototype can be scalability extrapolated if prototypes use factors such as cavitation and balance of fill (4-cavity mold or 4-fixture automation can be tolerance-scaled to 8, 16, 32, etc.).
Haney: For MTD, our ability to control degradation factors such as residual monomer count and molecular weight conservation allow us to preserve a material’s integrity like never before. This means we can take on more challenging part geometries, fill more intricate features, and ultimately expand what is possible to micro mold.
Herbert: Today’s materials are extremely flexible in their composition, so if something off the shelf isn’t working something custom can work. It all comes down to time and money, how much time and how fast do you want it.
Johnson: At Accumold we have a very strong relationship with our resin suppliers. We work closely with many of them, especially when they’re looking to test new materials. Currently we see a lot of interest in thermoplastics that can withstand high heat deflection, for example, a solder reflow process. The same demand we find with consumer electronics for smaller form factors is making its way into the medical device industry. The consolidation efforts around shrinking these devices has created an interesting challenge for the industry.
Passetti: Material advancements aren’t specific to micro-molding.
Barbella: How are less invasive procedures and point-of-care (POC) applications impacting micromolded device design and development?
Bibber: POC devices require compliance (ease of use, least pain to the patient). Tiny parts, needles, septums, sheaths, and tiny assembled devices are more helpful with human factors, identifying tiny features and protecting them in customized packages with humidity, temperature, and numerical and visual aids to help the user use the device properly. Small arteries, veins, valves, and small differences in forces from skin, patient blood, and skin chemistries all play a special role in adhesion, hydrophobicity, and device function. Testing for all types of human factors will prevent costly failures later on in the product development and clinical testing cycles.
Gerding: There is no micromolded device design. Micromolding is one answer to the general miniaturization trend. As devices get smaller, components have to follow. The next step will be to provide the same level of functional integration we offer with larger molded parts, as we do with overmolding, 2-shot molding, direct bonding, surface textures, etc.
Herbert: They’re not affecting our applications. It really comes down to tooling and cost to produce, how efficient are you and how much waste is there.
Gary Hulecki: Instead of molding standalone parts, gluing, and then assembling, which can produce failure modes and a level of uncertainly, precision overmolding has become a popular solution to these issues. Overmolding and fully encapsulated electronics for implants like batteries, circuit boards. This is possible with micromolding technology without risking damage or reducing functionality.
Unconventional approaches to fill and encapsulate these materials to protect the devices from high temperatures and high forces seen in molding.
Johnson: We’ve seen a large increase in the demand for micromolded components in the medical device industry. Certainly, the drive for better patient care, faster recoveries, and more advanced techniques have been accomplished through microelectronics and miniaturization. In addition, wearable technologies have come on the scene strongly as well. Whether it’s drug delivery or diagnostics, the closer something is to the patient oftentimes the better the care. We take a lot of pride at Accumold knowing we’re helping bring the best patient care possible to those in need.
Passetti: As surgeries and procedures become less and less invasive, the size of the devices have become smaller and smaller. We have seen this really grow in the past five to 10 years.
Schwenker: The continued trend of less invasive procedures in conjunction with the growth of micromolding is allowing more intricate devices to be developed for specific treatments. There is significant innovation occurring where implants and other devices can now be made designed to fit on the end of a catheter or be implanted in the body with little discomfort.
Barbella: What are customers demanding or expecting in their micromolded products?
Bibber: Our customers drive technology and systems that make us a better supplier.
Smaller assemblies, thinner walls, robust DOEs and protocols, and combination devices that combine geometry, materials, and assemblies fitting on the point of a needle.
The size of automated assemblies are incredibly strong yet tiny, such as 0.001-inch guidewires, sensors, integrated circuitry, and overmolding onto circuit boards—all to better science and medicine.
Delivering medicine at the point of use, and treating the patient directly where it is needed vs. swallowing a pill that eventually gets to the location it is required. This requires extremely tiny devices and micron level tolerances.
John Clark: Smaller parts, thinner walls, sharper features—without narrowing material choices. Customer demands also require molders to push limits of polymers. Achieving thinner walls than what is traditionally doable. For example, a customer may request for parts with .003-.004-inch walls in polycarbonate material. Manufacturer specifications for PC indicate that a tool should be vented up to .003 inches deep for this material. So, you are essentially molding flash at that point.
Gerding: Literally everything you would expect from a large part is required in micromolding. No one says, “you can’t see this with the naked eye so it might not be important.” Everything needs to be under control as they are with the molding of bigger parts.
Herbert: Always testing the envelope and certainly the tiniest dimensions.
Johnson: There’s a reason someone comes to Accumold for micromolded components. The parts and components that we work on are what we call “critical components.” They have tight tolerances and tough geometries, often made with expensive engineered thermoplastic resins. Our customers expect high output, with high consistency, and high efficiencies. The parts we produce are too important to leave to chance. That’s why we take great care from the very beginning of the process all the way through delivery to ensure customer satisfaction.
Lindsay Mann: Consistency and supplier consolidation. Having a one-stop shop for as many processes as possible is in high demand—tooling, validation, assembly, packaging, testing, secondary operations.
Passetti: Defect-free and on time delivery of parts.
Schwenker: Customers continue to expect precision molding and the quality systems in place. There is a greater awareness that micromolding requires precision beyond the mold itself, and that inspection of parts, detailed maintenance of the equipment, and handling systems all need to be part of the validated process. It is important for customers to understand that the material cost itself is almost irrelevant in micromolding, and that in many cases the actual infrastructure surrounding the process is a significant portion of the final part price.
Barbella: How is the changing regulatory environment affecting micromolding (medical devices)?
Bibber: As devices are used more point of use, regulatory compliance such as bioburden levels, particulate, surface finish, and material testing methods must all follow suit. These regulatory standards then spill into fully automated, in-line 100 percent visual and dimensional inspection. Industry 4.0 is required to address big data needs, cataloguing lot control to the individual and RFID component being manufactured. All of this compliance and data is used for continuous improvement and close loop information for next-generation devices. CT scanning creates STL files that are saved indefinitely. The parts themselves may be affected by shelf-life and environment; however the STL point clouds remain intact indefinitely. Using these files in an Industry 4.0 system helps medical and drug delivery device designers to create next-generation devices and assemblies by using this historical and extremely accurate and robust data files.
Herbert: It creates more documentation but does not affect the actual process.
Johnson: We’ve seen some changes that have made their way down to the component level of medical device manufacturing. We work closely with our customers to make sure things like lot control, the validation processes, or other certifications meet the demands they need for their industry compliance. The biggest change we’ve seen in recent times has more to do with the reimbursement side of the medical device industry. Changes in how medical device OEMs can sell their devices has put a lot of cost pressures on the supply chain to make up some of this lost ground.
Passetti: They are reclassifying a lot of devices to give the FDA more control over their use. There is a higher level of documentation and a higher level of repeatability.
Schwenker: As with injection molding, we continue to see a greater reliance on suppliers to be registered with the FDA, and to be a critical part of the supply chain, but this is not specific to micromolding.
Barbella: Are there any other trends you are noticing in micromolded device design and development?
Clark: Customers want turnkey systems and solutions. The trend shows a desire to continue to consolidate their supply chains.
Gerding: In micromolding you need to pay particular attention to the entire system, including tooling. Not every tool shop has the ultra-precision equipment or skills necessary to fabricate molds with micro features such as micro milling and laser structuring. Precision equipment needs to be in the nanometer range.
Herbert: We are seeing a 1,000 increase in opportunities as the world is getting smaller and smaller! “Get Small” has been our slogan for the last 16 years, and it’s happening day in and day out.
Hulecki: In-vitro diagnostics and the shift toward point of care will produce a growing need for micromolded components. There is a cost benefit to having a single source provider to make most components of a device and provide special processes like handling electronics and packing and shipping to the end user.
Johnson: Overmolding or insert molding are of high interest these days. When you can consolidate multiple components, you can pick up not only manufacturing efficiencies but sometimes reduced form factors. This process can also produce components that might otherwise be cost prohibitive to make. At Accumold we have overmolded all sorts of interesting media like glass, fabric, flex circuit, metals, and other plastics. We recommend always looking for ways to combine components when looking at your device design.
Passetti: Designers of micromolded device components are always pushing the envelope with regards to innovative designs and functions. Simulation has advanced in its capabilities of accurately predicting the processing outcomes of micromolded parts.
Schwenker: Overmolding and microassembly of micromolded parts appears to be a growing trend, which is allowing for greater functionality of these components.
Barbella: Where is micromolding headed in the next few years? What new technologies or capabilities are on the horizon?
Cicio: Over the next few years, micromolding will continue to grow and challenge the limits of mold makers and processors. Micromolders will continue to push the limits of materials and their abilities to fill thin-walled cavities, form sharp features, all while meeting tight tolerance and holding up to extremely high shear rates and maintaining the material’s desired mechanical properties.
Clark: CT scanning to measure smaller parts and features will become a requirement. Having this service in-house at molding facilities will become more of a requirement as well.
Gerding: We see standardization, auxiliary equipment, and process combinations (2-shot and overmolding).
Haney: It seems as the industry continues to realize the full potential that comes from the precision of the micromolding industry, we are beginning to realize that these micro components can interface with modern technology and electronics in ways thought to be impossible just years ago. As time goes on, I think we will see more and more micro medical components interfacing with smart technology in particular.
Herbert: Machine capabilities will be challenged along with cavitation; improving quality and quantity is imperative.
Hulecki: CT scanning provides the ability to scan/measure an entire assembled device vs. individual parts of a device. Artificial intelligence coming into injection molding—micromolding too. Use AI to simplify the molding process/setup—algorithms to calculate process parameters to create a robust molding process.
Johnson: As we continue to watch the demand for smaller and smaller products, we will see more innovation around micromolding processes like overmolding and insert molding, thin-wall capabilities, and the molding of other extreme geometries become more commonplace. These processes will be key factors in future product development. We tell all our customers, “design what you think you need, and let’s start the conversation there,” you never know what just might be possible.
Passetti: Advancements in machine technology and the methodology behind micromolding itself. Advancements in micro machining of tooling is out and on the horizon, too. Advancements in 3D printing are pushing the design and development timelines by compressing time to market, and new processing methodologies are on the horizon.
Schwenker: As the trend continues coupled with less invasive technology, we continue to believe that micromolding will become more accessible. Tool design will continue to play an important role in bringing more products to the market, and integrated automation and inspection systems will be required even at low volumes to meet the needs of the medical device market.
Those three words have become the unofficial motto of medtech development in recent decades as clinicians and innovators worked to improve both patient care and access. Driven by the advent of digital health and minimally invasive technologies, the slogan has spawned numerous inventions that are less intrusive and better suited for home use (think oxygen concentrators, CPAP machines, cochlear implants, and pacemakers).
“Medical manufacturers are moving entire systems—from home-based and clinical devices to imaging applications—into a portable unit the size of a cell phone or smaller,” states a 2016 whitepaper from Microsemi Corporation, an Aliso Viejo, Calif.-based provider of semiconductor and system solutions for the aerospace and defense, communications, data center, and industrial markets. “What was once huge equipment tethered to a wall has become available in mobile clinics, ambulances, and in a doctor’s bag for house calls.”
Some devices are now even small enough to be lost inside the doctor’s bag. Pacemakers, for example, have shrunk from the size of a cathode ray tube television set to that of a large pill. Medtronic plc spent a decade developing the one-inch Micra, a device small enough to be inserted through a groin vein and fed through the femoral artery to the heart. At one-tenth the size of traditional pacemakers, Micra is an upshot of Medtronic’s “deep miniaturization” program, an initiative that aims to shrink medical devices by up to 90 percent.
“We will no longer pace the heart in the way we have in the last 20 to 30 years,” John Hummel, M.D., a cardiologist who participated in Micra clinical trials, said when the device received U.S. Food and Drug Administration approval in April 2016. “This is fundamentally a paradigm shift in how we’ll deliver this therapy. We are looking at the beginning of the future.”
The key to ensuring that future is micromolding, a highly specialized manufacturing process used to create tiny, 3D components with small features and dimensional tolerances. Micromolded parts are made from various thermoplastic materials including PEEK (polyether ether ketone), liquid crystal polymer, and nylon, though components also can be comprised of optical and medical-grade materials. The types of substances used allow for mold sizes to be smaller than that of a dime.
To better understand the factors and trends driving the micromolding industry, Medical Product Outsourcing spoke with numerous industry professionals over the last month. They included:
- Donna Bibber, vice president of business development, and Wayne Shakal, vice president of operations at Isometric Micro Molding Inc., a New Richmond, Wis.-based micromolding firm specializing in micro injection molding, micro automation, and micro assembly.
- Jared Cicio, John Clark, Patrick Haney, Gary Hulecki, Lindsay Mann, and Peter Wojtas of MTD Micro Molding, a Charleton, Mass.-based micro medical manufacturer of ultra-precision molded components. Cicio is production supervisor of molding; Clark is operations manager; Haney is an R&D engineer; Hulecki is an executive vice president; Mann is sales and marketing director; and Wojtas is a senior process engineer.
- Lars Gerding, technology director at Freudenberg Medical, a developer and manufacturer of micro components in thermoplastics and PEEK. The company also offers highly specialized micromolding and micro extrusion services in silicone and implantable silicone.
- Scott Herbert, president and founder of Rapidwerks Inc., a Pleasanton, Calif.-based precision micromolder.
- Aaron Johnson, vice president of marketing and customer strategy at Accumold, an Ankeny, Iowa-headquartered high-tech manufacturer of precision micro, small, and lead frame injection-molded plastic components.
- Ben Passetti, an R&D engineer at Tessy Plastics, a molder and contract manufacturer specializing in high-speed, multi-shot, and thin-wall injection molding and micromolding. Based in Elbridge, N.Y., the firm integrates molding with assembly, decorating, and testing operations.
- Rob Schwenker, vice president of sales, Specialty Molding, at Spectrum Plastics. Headquartered in Atlanta and with multiple plants in North America and beyond, the Spectrum Plastics Group designs, develops, and manufactures specialty medical plastic products.
Michael Barbella: What are the latest advancements in micromolding technologies?
Lars Gerding: We see more and more standardization around the demolding process and with inline quality inspection/vision control.
Patrick Haney: As of late, MTD has been taking a serious stance on understanding and manipulating our final products on a molecular level. With our constant effort to learn, we have launched a series of characterization projects to analyze the morphology of our molded components. Equipped with this knowledge, we are beginning to develop custom and original technology to incorporate into our injection molds to manipulate such things.
Scott Herbert: Significant advancements in control of materials.
Aaron Johnson: Like a lot of manufacturing services, micro molding innovation is driven by the demand of our customers pushing the limits of their own technology. In a lot of cases designs are asking to do more in the same space, or commonly, more in less. This demand puts a lot of pressure on the supply chain to constantly be looking for solutions. Advancements like extreme thin-wall molding, 2-shot micro molding, and automated insert molding are a direct result from the marketplace.
Ben Passetti: Injection molding equipment and technology in general. New micromolding sensors have come a long way and have been made specifically to fit the molds (the sensors used to be too big).
Rob Schwenker: Advancements in micromolding for Spectrum Plastics continue to be focused on automated part handling and measurement systems. The ability to mold parts is only as good as your ability to prove that your precision is accurate. It is also important to build measurement systems early on during the development phase as it is during validation.
Wayne Shakal: Tooling: The advancement of 5-axis micro CNC machine tools and micro sinker EDM allowing for micron level detail and accuracy. Micromolding: New style injection units that enable molders to inject with sub-1 gram shot sizes with minimal degradation and very high shot-to-shot accuracy. The advancement in the miniaturization of pressure and temperature sensors is allowing the ability to better control processes and provide real-time monitoring and in some cases closed-loop process control. Validation: The advancement of in-house CT scanning and 3D laser scanning technologies have given us the ability to achieve passing gauge R&R’s to inspect plastic dimensions with micron tolerances.
Peter Wojtas: Runnerless or reduced flow path molds to conserve expensive materials will lead to machine manufacturers redesigning to achieve high precision with extremely small shot sizes. Other recent advancements include custom screw designs for exotic materials, improved reduced-wall-thickness-filling abilities, in-mold annealing and stress relief, and improved material and mold monitoring systems (smaller mold sensors, real-time moisture data).
Barbella: What challenges are associated with producing small, delicate, intricate micromolded components for medical devices? How does your company meet those challenges?
Jared Cicio: Handling and packaging can be challenging with small, delicate parts. Bulk packing micro parts can be the easiest method, but with small, intricate, sharp features, figuring out a way to get the parts from the molding machine to the customer can prove to be just as difficult as producing the part. More micro parts require individually packing in custom packaging or trays to protect the features and allow for easier handling. There are many other challenges that come with producing such small parts. Testing is performed to determine the safest temperature to keep machines at while material is in the barrel, but not running. We also have ionizing fans in place to avoid any static which can lead to airborne contamination attaching to molded parts. We also incorporate multiple covered drop zones that catch our parts and keep them safe from contamination until they are packaged.
Gerding: Standardization, dimensional control, in-line close loop feedback, and reproducibility with no rework required. In addition, part handling (demolding, bagging, etc.) of micro-sized silicone products is a challenge in itself.
Haney: The primary challenges involved with producing such small components can be summarized as “the need for absolute precision and attention to detail in every category imaginable.” When it comes to producing components that are intended to serve such a critical application, we need to make sure that we are controlling every aspect of the manufacturing process. This means everything from unprecedented precision in machining the molding components, to monitoring and controlling the final morphology of the molded product. Our top down facility allows us to achieve such feats by allowing MTD to keep every aspect of the product line in-house, from mold making, to processing, to inspection, and material characterization.
Herbert: The challenges are plentiful, they are all different and unique. Most are lumped in to just a few areas. Part handling, part orientation, and in some cases static, all of which are controlled by custom automation and advancements in static control.
Johnson: One of the biggest challenges in producing micro components for medical devices is making sure you have the systems in place for quality control, especially when dealing with tight tolerance plastic components. Building micro components with micron tolerances is difficult enough. The validation process for most medical device components is very demanding. At Accumold, we follow the ISO 13485 quality management process throughout the organization to ensure the product that we make meets the customer’s expectations. We also work very closely with our customers to understand their exact needs from the very beginning.
Passetti: Mold design and construction are critical. Achieving shot-to-shot consistency is one of the largest challenges that we face and this applies more to the processing/handling side of things.
However, it all depends on the machine and the pellets that you use along with the compression. These things determine the performance the most. We partner with some of the best tool shops in the world to overcome these challenges. We also have the capability in house to maintain the molds and a high level of tooling. We select some of the best machines in the world and test them rigorously, and modify them based on need and improved repeatability. The age of assets is less than five years old to keep relevant with technology—this improves efficiencies as time goes on.
Schwenker: Some of the most demanding challenges surround micromolding over inserts, the placement and management of inserts requires precise placement, or you risk tool damage. In many cases automation is not implemented until products have reached a certain volume threshold, so operators who are skilled at working under microscopes with calm controlled movements are highly valued.
Training programs around working under magnification and understanding operator’s skill sets in these areas can often be the reason for the success of a program. These same challenges apply to managing the quality and inspection of parts as well.
Shakal: Tooling: Isometric has invested in state-of-the-art micro machining and mold technology that allows us to produce part geometries that would be deemed impossible by most mold builders and molders. Micromolding: Coupled with our vast amount of micro-focused research and development, we’ve established various tooling and molding methods that give us additional fill capabilities. It almost always comes down to the ability to fill and the difference between failure and success is often less than .0001 grams (0.1 mg) of fill.
Barbella: What factors are typically taken into consideration when selecting materials for micromolded device components?
Donna Bibber: Materials are typically selected for their function first. Flexibility, rigidity, and strength.
The classification of device and whether there has been a predicate device using the actual grade of material. Also, is the material able to be manufactured/molded to the size, wall thickness ranges (melt flow rate, molecular weight distribution, fillers preventing flow, feature size replication)? Is the material lot-to-lot variation or cavity-to-cavity variation able to be extrapolated in scale to be within the acceptable Cpk range?
Gerding: The materials used in silicone micromolding are the same materials used in larger parts. The key to success lies in how the materials are handled and dispensed. You may think viscosity would play a role in order to fill small features along the melt flow path but silicone flows like water. Flash control is much more important in micromolding, as flash can be larger than the micro part.
Haney: There are many factors to consider during the material selection phase of a project. Through the manufacturing process all the way to application, each phase of a micromolded project presents its own set of unique aspects to consider. First and foremost, items like mechanical, thermal, and degradation mechanisms all must meet the requirements of the intended application. However, in addition to the standard “form, fit, and function” considerations, properties such a crystallinity, shear sensitivity, compressibility, and other rheological aspects become exponentially more important when scaling down to a micro-sized component. We must also make sure the material selected will conform to such requirements in order to manufacture a reliable product.
Herbert: Material selection is very critical and application-specific. Implants and resorbable materials are all dependent on the end use.
Johnson: It’s common to start the material selection process by looking at the inherent properties of the material itself. You might be looking for biocompatibility, heat resistance or other attributes the material might provide. The challenge comes when trying to match the material selection with the desired geometries. With micromolding the smaller the feature you’re looking to produce, the more material selection becomes a critical part of the process. For example, PEEK is a common material selected in the medical device industry but if you’ve ever worked with this particular resin it isn’t always friendly to micro features. Understanding the tool build and the processing side of micromolding becomes very important in the design for manufacturability. In these cases, choosing a molder with experience understanding resins and micro feature performance will be an advantage.
Passetti: Pellet size and consistency of the raw material are the two main factors that are taken into consideration when selecting materials for micromolded device components.
Schwenker: It is important to understand the rheology of the material and minimize material degradation. Because we are dealing with such small volumes of material, process techniques can have a significant impact on part integrity.
Barbella: What material advances are currently impacting micromolding capabilities?
Bibber: Particle size distribution (pellet size), and pre-compounding effectiveness, especially if specialty pantones are required.
Material melt flow ranges to be specified at purchase to address micron tolerance Cpk.
New materials used in prototyping methods (3D printing) can show false negatives that a product will not be reproducible. Material selection and scalability at the micro level requires using materials, processes, and manufacturing methods that are constant from prototype to pilot to production so learnings and test results do not require repeating. Assumptions made at prototype can be scalability extrapolated if prototypes use factors such as cavitation and balance of fill (4-cavity mold or 4-fixture automation can be tolerance-scaled to 8, 16, 32, etc.).
Haney: For MTD, our ability to control degradation factors such as residual monomer count and molecular weight conservation allow us to preserve a material’s integrity like never before. This means we can take on more challenging part geometries, fill more intricate features, and ultimately expand what is possible to micro mold.
Herbert: Today’s materials are extremely flexible in their composition, so if something off the shelf isn’t working something custom can work. It all comes down to time and money, how much time and how fast do you want it.
Johnson: At Accumold we have a very strong relationship with our resin suppliers. We work closely with many of them, especially when they’re looking to test new materials. Currently we see a lot of interest in thermoplastics that can withstand high heat deflection, for example, a solder reflow process. The same demand we find with consumer electronics for smaller form factors is making its way into the medical device industry. The consolidation efforts around shrinking these devices has created an interesting challenge for the industry.
Passetti: Material advancements aren’t specific to micro-molding.
Barbella: How are less invasive procedures and point-of-care (POC) applications impacting micromolded device design and development?
Bibber: POC devices require compliance (ease of use, least pain to the patient). Tiny parts, needles, septums, sheaths, and tiny assembled devices are more helpful with human factors, identifying tiny features and protecting them in customized packages with humidity, temperature, and numerical and visual aids to help the user use the device properly. Small arteries, veins, valves, and small differences in forces from skin, patient blood, and skin chemistries all play a special role in adhesion, hydrophobicity, and device function. Testing for all types of human factors will prevent costly failures later on in the product development and clinical testing cycles.
Gerding: There is no micromolded device design. Micromolding is one answer to the general miniaturization trend. As devices get smaller, components have to follow. The next step will be to provide the same level of functional integration we offer with larger molded parts, as we do with overmolding, 2-shot molding, direct bonding, surface textures, etc.
Herbert: They’re not affecting our applications. It really comes down to tooling and cost to produce, how efficient are you and how much waste is there.
Gary Hulecki: Instead of molding standalone parts, gluing, and then assembling, which can produce failure modes and a level of uncertainly, precision overmolding has become a popular solution to these issues. Overmolding and fully encapsulated electronics for implants like batteries, circuit boards. This is possible with micromolding technology without risking damage or reducing functionality.
Unconventional approaches to fill and encapsulate these materials to protect the devices from high temperatures and high forces seen in molding.
Johnson: We’ve seen a large increase in the demand for micromolded components in the medical device industry. Certainly, the drive for better patient care, faster recoveries, and more advanced techniques have been accomplished through microelectronics and miniaturization. In addition, wearable technologies have come on the scene strongly as well. Whether it’s drug delivery or diagnostics, the closer something is to the patient oftentimes the better the care. We take a lot of pride at Accumold knowing we’re helping bring the best patient care possible to those in need.
Passetti: As surgeries and procedures become less and less invasive, the size of the devices have become smaller and smaller. We have seen this really grow in the past five to 10 years.
Schwenker: The continued trend of less invasive procedures in conjunction with the growth of micromolding is allowing more intricate devices to be developed for specific treatments. There is significant innovation occurring where implants and other devices can now be made designed to fit on the end of a catheter or be implanted in the body with little discomfort.
Barbella: What are customers demanding or expecting in their micromolded products?
Bibber: Our customers drive technology and systems that make us a better supplier.
Smaller assemblies, thinner walls, robust DOEs and protocols, and combination devices that combine geometry, materials, and assemblies fitting on the point of a needle.
The size of automated assemblies are incredibly strong yet tiny, such as 0.001-inch guidewires, sensors, integrated circuitry, and overmolding onto circuit boards—all to better science and medicine.
Delivering medicine at the point of use, and treating the patient directly where it is needed vs. swallowing a pill that eventually gets to the location it is required. This requires extremely tiny devices and micron level tolerances.
John Clark: Smaller parts, thinner walls, sharper features—without narrowing material choices. Customer demands also require molders to push limits of polymers. Achieving thinner walls than what is traditionally doable. For example, a customer may request for parts with .003-.004-inch walls in polycarbonate material. Manufacturer specifications for PC indicate that a tool should be vented up to .003 inches deep for this material. So, you are essentially molding flash at that point.
Gerding: Literally everything you would expect from a large part is required in micromolding. No one says, “you can’t see this with the naked eye so it might not be important.” Everything needs to be under control as they are with the molding of bigger parts.
Herbert: Always testing the envelope and certainly the tiniest dimensions.
Johnson: There’s a reason someone comes to Accumold for micromolded components. The parts and components that we work on are what we call “critical components.” They have tight tolerances and tough geometries, often made with expensive engineered thermoplastic resins. Our customers expect high output, with high consistency, and high efficiencies. The parts we produce are too important to leave to chance. That’s why we take great care from the very beginning of the process all the way through delivery to ensure customer satisfaction.
Lindsay Mann: Consistency and supplier consolidation. Having a one-stop shop for as many processes as possible is in high demand—tooling, validation, assembly, packaging, testing, secondary operations.
Passetti: Defect-free and on time delivery of parts.
Schwenker: Customers continue to expect precision molding and the quality systems in place. There is a greater awareness that micromolding requires precision beyond the mold itself, and that inspection of parts, detailed maintenance of the equipment, and handling systems all need to be part of the validated process. It is important for customers to understand that the material cost itself is almost irrelevant in micromolding, and that in many cases the actual infrastructure surrounding the process is a significant portion of the final part price.
Barbella: How is the changing regulatory environment affecting micromolding (medical devices)?
Bibber: As devices are used more point of use, regulatory compliance such as bioburden levels, particulate, surface finish, and material testing methods must all follow suit. These regulatory standards then spill into fully automated, in-line 100 percent visual and dimensional inspection. Industry 4.0 is required to address big data needs, cataloguing lot control to the individual and RFID component being manufactured. All of this compliance and data is used for continuous improvement and close loop information for next-generation devices. CT scanning creates STL files that are saved indefinitely. The parts themselves may be affected by shelf-life and environment; however the STL point clouds remain intact indefinitely. Using these files in an Industry 4.0 system helps medical and drug delivery device designers to create next-generation devices and assemblies by using this historical and extremely accurate and robust data files.
Herbert: It creates more documentation but does not affect the actual process.
Johnson: We’ve seen some changes that have made their way down to the component level of medical device manufacturing. We work closely with our customers to make sure things like lot control, the validation processes, or other certifications meet the demands they need for their industry compliance. The biggest change we’ve seen in recent times has more to do with the reimbursement side of the medical device industry. Changes in how medical device OEMs can sell their devices has put a lot of cost pressures on the supply chain to make up some of this lost ground.
Passetti: They are reclassifying a lot of devices to give the FDA more control over their use. There is a higher level of documentation and a higher level of repeatability.
Schwenker: As with injection molding, we continue to see a greater reliance on suppliers to be registered with the FDA, and to be a critical part of the supply chain, but this is not specific to micromolding.
Barbella: Are there any other trends you are noticing in micromolded device design and development?
Clark: Customers want turnkey systems and solutions. The trend shows a desire to continue to consolidate their supply chains.
Gerding: In micromolding you need to pay particular attention to the entire system, including tooling. Not every tool shop has the ultra-precision equipment or skills necessary to fabricate molds with micro features such as micro milling and laser structuring. Precision equipment needs to be in the nanometer range.
Herbert: We are seeing a 1,000 increase in opportunities as the world is getting smaller and smaller! “Get Small” has been our slogan for the last 16 years, and it’s happening day in and day out.
Hulecki: In-vitro diagnostics and the shift toward point of care will produce a growing need for micromolded components. There is a cost benefit to having a single source provider to make most components of a device and provide special processes like handling electronics and packing and shipping to the end user.
Johnson: Overmolding or insert molding are of high interest these days. When you can consolidate multiple components, you can pick up not only manufacturing efficiencies but sometimes reduced form factors. This process can also produce components that might otherwise be cost prohibitive to make. At Accumold we have overmolded all sorts of interesting media like glass, fabric, flex circuit, metals, and other plastics. We recommend always looking for ways to combine components when looking at your device design.
Passetti: Designers of micromolded device components are always pushing the envelope with regards to innovative designs and functions. Simulation has advanced in its capabilities of accurately predicting the processing outcomes of micromolded parts.
Schwenker: Overmolding and microassembly of micromolded parts appears to be a growing trend, which is allowing for greater functionality of these components.
Barbella: Where is micromolding headed in the next few years? What new technologies or capabilities are on the horizon?
Cicio: Over the next few years, micromolding will continue to grow and challenge the limits of mold makers and processors. Micromolders will continue to push the limits of materials and their abilities to fill thin-walled cavities, form sharp features, all while meeting tight tolerance and holding up to extremely high shear rates and maintaining the material’s desired mechanical properties.
Clark: CT scanning to measure smaller parts and features will become a requirement. Having this service in-house at molding facilities will become more of a requirement as well.
Gerding: We see standardization, auxiliary equipment, and process combinations (2-shot and overmolding).
Haney: It seems as the industry continues to realize the full potential that comes from the precision of the micromolding industry, we are beginning to realize that these micro components can interface with modern technology and electronics in ways thought to be impossible just years ago. As time goes on, I think we will see more and more micro medical components interfacing with smart technology in particular.
Herbert: Machine capabilities will be challenged along with cavitation; improving quality and quantity is imperative.
Hulecki: CT scanning provides the ability to scan/measure an entire assembled device vs. individual parts of a device. Artificial intelligence coming into injection molding—micromolding too. Use AI to simplify the molding process/setup—algorithms to calculate process parameters to create a robust molding process.
Johnson: As we continue to watch the demand for smaller and smaller products, we will see more innovation around micromolding processes like overmolding and insert molding, thin-wall capabilities, and the molding of other extreme geometries become more commonplace. These processes will be key factors in future product development. We tell all our customers, “design what you think you need, and let’s start the conversation there,” you never know what just might be possible.
Passetti: Advancements in machine technology and the methodology behind micromolding itself. Advancements in micro machining of tooling is out and on the horizon, too. Advancements in 3D printing are pushing the design and development timelines by compressing time to market, and new processing methodologies are on the horizon.
Schwenker: As the trend continues coupled with less invasive technology, we continue to believe that micromolding will become more accessible. Tool design will continue to play an important role in bringing more products to the market, and integrated automation and inspection systems will be required even at low volumes to meet the needs of the medical device market.