Sam Brusco, Associate Editor04.03.19
Transitioning from reactive to proactive/preventive care could work wonders for the healthcare system. A proactive strategy is important for cardiovascular disease, the leading cause of death across the globe.
According to a 2017 American Heart Association (AHA) report, heart failure (HF) affects about 6.5 million Americans annually, with almost 1 million new cases every year.
Addressing HF before it lands patients in the hospital can also reduce America’s already troublesome healthcare spending. The AHA reports that HF costs the U.S. about $30.7 billion annually, and that spend is expected to rise 127 percent to $69.7 billion by 2030. Further measures must be taken to reduce HF patients’ hospital readmissions because hospitalization accounts for about 80 percent of HF’s total cost.
To keep HF patients out of the hospital, more interventions aimed at identifying and monitoring sub-clinical heart congestion can boost chronic HF management at home. Such home management includes self-care and home visitations, and the use of telemedicine and remote monitoring via external or implantable devices.
Your toilet, for example.
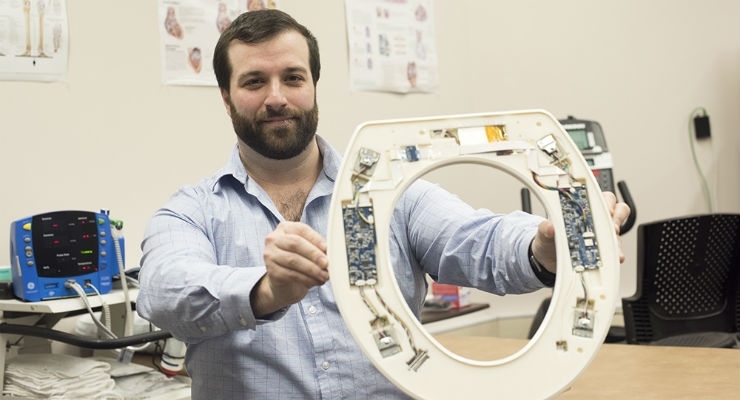
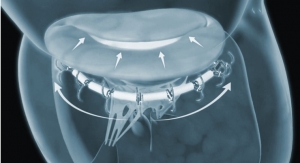
No, this isn’t an April Fool’s prank. Rochester Institute of Technology (RIT) researchers have created a toilet-seat based cardiovascular monitoring system with the goal of reducing hospital readmission rates for HF patients. The high-tech add-on to the porcelain throne features built-in biometric sensors that measure heart rate, blood pressure, blood oxygenation levels, weight, and stroke volume—the amount of blood pumped at each heartbeat. The system’s specialized algorithms analyze the biometric data, and upon further development, alert healthcare providers of a deteriorating condition. Cardiologists can then read the report to decide whether intervention is necessary.
“Typically, within 30 days of hospital discharge, 25 percent of patients with congestive heart failure are readmitted,” said Nicholas Conn, a postdoctoral fellow at RIT and founder and CEO of Heart Health Intelligence, who is part of the university team that developed the toilet seats. “After 90 days of hospital discharge, 45 percent of patients are readmitted. And the Centers for Medicare and Medicaid Services is penalizing hospitals for readmitting patients for heart failure.”
And though the idea seems ludicrous, the toilet makes sense as a periodic heart health checkpoint. The toilet seat system can be easily integrated into a monitoring regimen with quite literally no change to daily routine because patients (with healthy digestion) are guaranteed to sit down for a heart checkup at least once a day. Subject preparation and error problems are lessened because skin contact is automatic and very repeatable for each use—there are few actions more innate than a trip to the john.
RIT’s proposed cardiovascular monitoring system installs directly on a standard toilet, is battery powered, wireless, waterproof, and doesn’t need further connections or user interaction. The seat’s single lead ECG measures the heart’s electrical activity, a ballistocardiogram measures the heart’s mechanical forces, and a photoplethysmogram measures blood oxygenation and pulse transit time.
The toilet seats are slated for the FDA clearance process by the researchers’ company Heart Health Intelligence. Once cleared, the toilet seats are intended for purchase by hospitals and would be given to HF patients following discharge. The team is currently writing grants for further funding and networking, and have begun human subject testing and preclinical studies.
One significant issue currently clogging the pipes is that the toilet-based monitor doesn’t work as one is relieving oneself. Unfortunately, using the toilet for its intended purpose befouls the measurements, as well. The researchers aim to repair this, writing in an application for the device that “in future in-home studies, algorithms will be developed to identify and reject periods of urination and defecation through classification of motion artifacts and the physiologic shifts associated with this change in state.”
The heart-healthy toilet seat can be viewed as the latest—and most creative—variation on the trendy theme of wearable medical devices. It fits with the idea of tracking health whenever and wherever possible, which is of the utmost importance for cardiac health, where an event can strike at any moment without much warning.
The toilet seat plays a similar role to consumer technology with a healthy twist, much like the massively popular Apple Watch. Apple recently released the results of a 400,000-patient trial evaluating the Watch’s efficacy in accurately detecting arrhythmias at this year’s annual meeting of the American College of Cardiology. Participants wore the Apple Watch and were sent a notification if the smartwatch spotted an irregular heartbeat, then followed up with an ECG for verification.
The Apple Heart Study did have a high rate of false positives (84 percent of the irregular pulse notifications were later confirmed as AFib episodes), which raises concerns about patients being treated unnecessarily or prematurely. However, the study results give Apple a reason to continue this type of study, primarily because the study didn’t use the company’s new Series 4 Watch, whose built-in ECG required FDA clearance. The toilet, as an experimental method to measure biometrics involved in HF, faces similar issues. However, it is important to note that these technologies should be viewed as an adjunct to a clinical assessment.
“The physician can use the information from the study, combine it with their assessment…and then guide clinical decisions around what to do with an alert,” Marco Perez, an associate professor of cardiovascular medicine at Stanford and one of the study’s lead researchers, told Reuters.
According to a 2017 American Heart Association (AHA) report, heart failure (HF) affects about 6.5 million Americans annually, with almost 1 million new cases every year.
Addressing HF before it lands patients in the hospital can also reduce America’s already troublesome healthcare spending. The AHA reports that HF costs the U.S. about $30.7 billion annually, and that spend is expected to rise 127 percent to $69.7 billion by 2030. Further measures must be taken to reduce HF patients’ hospital readmissions because hospitalization accounts for about 80 percent of HF’s total cost.
To keep HF patients out of the hospital, more interventions aimed at identifying and monitoring sub-clinical heart congestion can boost chronic HF management at home. Such home management includes self-care and home visitations, and the use of telemedicine and remote monitoring via external or implantable devices.
Your toilet, for example.
No, this isn’t an April Fool’s prank. Rochester Institute of Technology (RIT) researchers have created a toilet-seat based cardiovascular monitoring system with the goal of reducing hospital readmission rates for HF patients. The high-tech add-on to the porcelain throne features built-in biometric sensors that measure heart rate, blood pressure, blood oxygenation levels, weight, and stroke volume—the amount of blood pumped at each heartbeat. The system’s specialized algorithms analyze the biometric data, and upon further development, alert healthcare providers of a deteriorating condition. Cardiologists can then read the report to decide whether intervention is necessary.
“Typically, within 30 days of hospital discharge, 25 percent of patients with congestive heart failure are readmitted,” said Nicholas Conn, a postdoctoral fellow at RIT and founder and CEO of Heart Health Intelligence, who is part of the university team that developed the toilet seats. “After 90 days of hospital discharge, 45 percent of patients are readmitted. And the Centers for Medicare and Medicaid Services is penalizing hospitals for readmitting patients for heart failure.”
And though the idea seems ludicrous, the toilet makes sense as a periodic heart health checkpoint. The toilet seat system can be easily integrated into a monitoring regimen with quite literally no change to daily routine because patients (with healthy digestion) are guaranteed to sit down for a heart checkup at least once a day. Subject preparation and error problems are lessened because skin contact is automatic and very repeatable for each use—there are few actions more innate than a trip to the john.
RIT’s proposed cardiovascular monitoring system installs directly on a standard toilet, is battery powered, wireless, waterproof, and doesn’t need further connections or user interaction. The seat’s single lead ECG measures the heart’s electrical activity, a ballistocardiogram measures the heart’s mechanical forces, and a photoplethysmogram measures blood oxygenation and pulse transit time.
The toilet seats are slated for the FDA clearance process by the researchers’ company Heart Health Intelligence. Once cleared, the toilet seats are intended for purchase by hospitals and would be given to HF patients following discharge. The team is currently writing grants for further funding and networking, and have begun human subject testing and preclinical studies.
One significant issue currently clogging the pipes is that the toilet-based monitor doesn’t work as one is relieving oneself. Unfortunately, using the toilet for its intended purpose befouls the measurements, as well. The researchers aim to repair this, writing in an application for the device that “in future in-home studies, algorithms will be developed to identify and reject periods of urination and defecation through classification of motion artifacts and the physiologic shifts associated with this change in state.”
The heart-healthy toilet seat can be viewed as the latest—and most creative—variation on the trendy theme of wearable medical devices. It fits with the idea of tracking health whenever and wherever possible, which is of the utmost importance for cardiac health, where an event can strike at any moment without much warning.
The toilet seat plays a similar role to consumer technology with a healthy twist, much like the massively popular Apple Watch. Apple recently released the results of a 400,000-patient trial evaluating the Watch’s efficacy in accurately detecting arrhythmias at this year’s annual meeting of the American College of Cardiology. Participants wore the Apple Watch and were sent a notification if the smartwatch spotted an irregular heartbeat, then followed up with an ECG for verification.
The Apple Heart Study did have a high rate of false positives (84 percent of the irregular pulse notifications were later confirmed as AFib episodes), which raises concerns about patients being treated unnecessarily or prematurely. However, the study results give Apple a reason to continue this type of study, primarily because the study didn’t use the company’s new Series 4 Watch, whose built-in ECG required FDA clearance. The toilet, as an experimental method to measure biometrics involved in HF, faces similar issues. However, it is important to note that these technologies should be viewed as an adjunct to a clinical assessment.
“The physician can use the information from the study, combine it with their assessment…and then guide clinical decisions around what to do with an alert,” Marco Perez, an associate professor of cardiovascular medicine at Stanford and one of the study’s lead researchers, told Reuters.