Mark Crawford, Contributing Writer01.30.19
Many of the technology advances in the medical device market are electronics-related, driven by medical device manufacturers (MDMs) wanting to take advantage of fast-growing, data-driven consumer markets by building real-time multifunctionality into their products. This push is led by the digital health market (especially point-of-care devices and products), which is expected to grow in value from about $5 billion currently to over $90 billion by 2025. As devices become smaller, more functional, and lighter weight, so must their electrical components, including batteries. Next-generation electronics must occupy less space and fit into more challenging geometries. Many applications, especially wearables, require electronics that are flexible, or can even stretch. These modern needs have compelled many electronics manufacturing services (EMS) companies to evolve beyond simply offering manufacturing services to include medical product design, logistics, fulfillment, and repair.
A variety of evolving technology and business trends—including regulatory—are impacting the EMS landscape. For example, tax policy, tariffs, and new trade agreements are serious cost considerations MDMs must factor into medical device design and production. Regulatory requirements are also changing the relationships between MDMs, component suppliers, and service providers.
As a result, OEMs are re-evaluating their sourcing strategies and supply chain structure, looking for vertically integrated EMS suppliers that can help them reduce costs and navigate the complex and sometimes confusing regulatory processes. When they find the right partner, OEMs outsource their manufacturing and engineering needs to take advantage of the EMS vendor’s existing systems, facilities, and knowledge. Some EMS companies even provide complete global supply chain management and advanced quality systems, which may be more sophisticated than those of the OEM.
“The biggest trend we see is the need for EMS companies to be a valued business partner, as well as a manufacturing partner,” said Rick McClain, president of Milwaukee Electronics, a Glendale, Wis.-based provider of circuit board design and printed circuit board (PCB) assembly. “Tariffs and materials constraints are business challenges that require a team approach to address. EMS materials expertise, systems, facility options, and real-time communications capability are critical in this effort.”
What OEMs Want
OEMs are eager to design and manufacture smaller and smarter medical devices that incorporate Internet of Things (IoT) features. The capabilities of new sensor technologies continue to push the limits of design and manufacturing processes for electronics-embedded devices and equipment.
“OEMs are asking for more predictability, reliability, and flexibility out of their technologies, all while improving time to market and reducing cost,” said Lars Runquist, vice president of product development for Redgroup, a Minneapolis, Minn.-based contract manufacturer that develops and manufactures medical devices. “In response, contract manufacturers are becoming more integrated with production development, leveraging the latest technologies and skill sets.”
One way to keep costs down is by turning existing products into smart connected devices.
“There is a big demand for IoT solutions on devices that are already on the market, but have yet to be digitally connected,” said Walt Maclay, president of Voler Systems, a Sunnyvale, Calif.-based contract engineering firm that focuses on the design of wearable and IoT devices. “For example, orthopedic devices that have had no electronics can now be equipped with sensors and provide wireless communication. This can include information on the wear of joints, strain on the structure, or identification of the serial number using RFID [radio frequency identification].”
“Sometimes customers want to retrofit connectivity into an existing product, with the challenge of designing it in without affecting the patient’s familiarity with the current product and branding,” added Chris Conger, director of connected health technology for Phillips-Medisize, a Hudson, Wis.-based outsource provider of design development and manufacturing to the medical and pharmaceutical markets. “Others are looking for a new product or platform, often worn on the body, requiring thinner, flexible, smaller designs.”
OEMs expect medical EMS companies to deliver advanced medical manufacturing capabilities, including compliance management, automation, and data analytics. Increasingly, OEMs expect their EMS partners to solve manufacturing problems they cannot solve themselves—this is typically accomplished during the design for manufacturability (DFM) process. OEMs seek EMS services that can deliver mechatronic solutions that seamlessly integrate with the rest of the system. “These solutions include modified mechanical components, custom electronics design, special communication interfaces, and the manufacturing of the solution in compliance with the regulatory requirements,” said Christian Fritz, director of sales for motion control and electronics for maxon precision motors, a Fall River, Mass.-based provider of high-precision drive systems, including motor control electronics.
New Trends and Technologies
Flexible electronics is a segment of the market especially key for miniaturizing devices. They are essential for creating complex devices that can fit into small physical spaces, such as devices deployed within the human body. Many new flexible (and even stretchable) sensors are coming on the market, which can measure parameters such as stretching, angle, and force, all of which are important for flexible devices. Other flexible sensors can measure touch, compression, and pressure, while also detecting variances in performance.
Wearable medical devices, especially, are driving the need for flexible, smaller, lighter, and easier-to-use products. Key production technologies include miniaturized chip scale packaging, molded interconnect devices that integrate plastic and electronic components, and flexible electronics (copper and polyester films that have electronics printed on them with roll-to-roll processing). “Although these technologies require special equipment and manufacturing expertise, they give designers a wider window to deliver innovations that potentially improve patient comfort, compliance, and health,” said Conger.
In recent years, new processes have been developed that can manufacture flexible electronics in volume. “Some companies can make the integrated circuits or chips flexible by grinding them down to be very thin, and they can deposit flexible passive components—in fact, an entire electronic circuit can now be made flexible,” said Maclay.
Assembly technology, overall, has not seen major changes in the last few years; technological advancements are predominately in components and printed circuit board design. “From an EMS standpoint, the drivers of change would be the re-spin of ISO 13485, as well as several other quality systems, to drive additional focus on risk analysis and mitigation, a materials market with constraints that drive a need for better long-term planning, and tighter regulatory controls driving the need for more detailed quality data collection and device history record keeping,” said McClain.
Along these lines, more companies are investing in systems technologies that support real-time data collection and analysis while also improving communications with customers on issues related to material availability and product lifecycle planning. New developments in IoT, machine-to-machine communications, and the cloud (all of which depend upon electronics and sensors) have emerged that are helping solve complex manufacturing problems.
“These also provide more visibility across the global supply chain and enable quality teams to more quickly and efficiently ‘mistake proof’ production processes, so the most common causes of manufacturing error can be avoided,” said Charlie Mason, senior vice president of the medical market segment for Sanmina, a San Jose, Calif.-based provider of medical imaging systems and diagnostics equipment.
According to Susan Mucha, president of Powell-Mucha Consulting, an El Paso, Texas-based consulting firm that works with EMS providers, the biggest assembly challenge is material constraints. “Systems connectivity within the factory seems to be improving, but when you have a supply chain where confirmed orders can be cancelled the day they are scheduled to ship, the best systems in the world don’t change that,” she said. “Old-fashioned human intervention is often the only way to fix it. If the issues creating shortages in the components market can be resolved, [i.e., industry consolidation that cuts capacity, unanticipated demands for passives and some memory types, longer lead times], I think we will see a big increase in efficiency in the EMS industry, especially in smaller EMS companies.”
Electronic components can now be 3D-printed, which provides potential for shortening the product development times for electronics—a key step toward reducing the bottleneck in product development and keeping electronic development in pace with mechanical development. “We can design, print, and test a mechanical component multiple times in the same day with existing rapid prototyping technologies, including 3D printing,” said Runquist. “If electronics can continue to improve towards those timelines, we can validate our final products faster and get to market sooner.”
One of the most recent advances in 3D printing was accomplished by the University of Washington (UW) in collaboration with SIJ Technology and Japan’s National Institute of Advanced Industrial Science and Technology. UW purchased and installed an ultra-high-resolution electronics printer and integrated it with a roll-to-roll printer, making it the first system capable of high-throughput printing at sub-micron feature sizes. The printer will be available for academic and industry research and development, prototyping, and commercial manufacturing.
This system will be especially helpful in developing 3D-printed next-generation electronics that are sustainably manufactured with earth-abundant materials. Electronic devices such as touchscreens and other displays typically depend on rare earth and scarce materials that are transparent and electrically conductive, like indium tin oxide (ITO). According to UW, modeling has shown that electrodes made of earth-abundant materials (and therefore cheaper) can be patterned with micron-scale features, making them competitive with ITO electrodes. Copper-based transparent electrodes with nanoscale features, for example, could actually match or exceed the conductivity and transparency of conventional ITO electrodes.
“Our users will be able to print electronics using sustainable materials with finer control than ever before, and it will directly enable UW and industrial researchers to develop a sustainable alternative for a crucial element of flexible thin-film solar cells, displays, and touch screens,” said J. Devin MacKenzie, a University of Washington materials science mechanical engineering associate professor. “This printer, the first of its kind in the world, can also be used to make improved sensors and higher power batteries.”
Internet of Things
OEMs are constantly pushing for connectivity. EMS companies are being approached by customers who are eager to leverage IoT technologies (e.g., communication interfaces, security, and increased data collection and analytics) to broaden their services offerings in hot fields such as wearables. Major challenges, however, are security and data protection. The immediate and overwhelming demands by OEMs to build communications capabilities into so many devices creates intense design and production pressures on their EMS partners, who may not be experts with some of the communications technologies involved.
“There is a big push for IoT and tying products to customer’s cell phones,” said Thomas Allen, vice president of sales and marketing for TRICOR Systems, an Elgin, Ill.-based electronic contract manufacturer for the medical device industry. “While this seems like a good idea, it can add additional costs, risk, and security concerns. One example of these costs is that an OEM’s phone application software will have to support and keep current with multiple operating systems.”
In terms of operations, IoT and the cloud have an increasing impact on the factory floor and the entire supply chain. For example, five years ago, Sanmina realized that deploying its manufacturing execution system (MES) in the cloud to connect 60 factories and 25,000 pieces of manufacturing equipment had the potential to provide real-time supply chain visibility in ways not possible before. It created a “digital twin” of each factory in the cloud, with systems to collect and analyze critical manufacturing data. The connectivity of physical factories to a cloud platform enables real-time global visibility of manufacturing, as well as the ability to alert operators and engineers when human intervention is required. It also provides a mechanism for the management of supply chains across organizational boundaries.
“This technology provides real value to a chief operating officer having responsibility for 20 global factories,” said Mason. “When traveling, he or she can receive real-time notifications of problems in any of these factories, based on pre-programmed alert levels, or for products related to a particular customer. This same technology also provides real-time global supply chain visibility to a chief product officer.”
Regulatory Landscape
Regulatory requirements continue to change. This is especially true for devices deployed into the European market, where the new Medical Devices Regulation (MDR) has added many changes, including efficacy as a requirement. Most new devices must comply with the new regulations if they are placed on the market after May 2020 and existing devices must meet the new regulations after May 2025. Although this makes the regulations in Europe and the United States more harmonized, it also makes Europe a less desirable place to launch new products.
With more wireless and wired communication features being added to medical electronics devices, and increased scrutiny by regulatory bodies, “there is a now more of a ‘show me’ factor in process development,” said McClain. “Customers don’t just want to hear the process is good. They want to see a statistical sampling plan that proves the process is good. This is a result of regulatory bodies asking them for similar data.”
FDA quality requirements continue to evolve. For some products, the FDA (or the OEM) requires the tracking of every component, every piece of manufacturing equipment, and all operator activity related to the creation of a medical device throughout the manufacturing process. Therefore, EMS providers need to document production at a much higher level than they did just a few years ago. For example, when the FDA audits a manufacturing facility, it is critical for that company to produce correct, clear, and verifiable quality records in a timely manner. This is best accomplished using electronic acquisition and storage of records.
Sanmina has applied cloud and other technologies to create what it calls a medical “forced quality framework.” Equipment and material as well as production operators are connected via bar code scans to a single database in the cloud. If a component is scanned prior to assembly and is the wrong part or the wrong revision, the system will prevent it from being used. If an operator is not trained according to a recent change in work instructions, he or she will be prevented from logging onto that workstation and performing the activity until properly trained. If an assembly somehow arrives at the wrong step on the production line, it will automatically be rejected.
“A complete record of all operator activity, tests, process data, and component traceability data exists in this cloud database and is linked to electronic device history records [eDHR] for comprehensive digital record keeping,” said Mason. “If a production error is found down the road, the database can be easily searched to isolate production issues, minimizing the unnecessary recall of good products. During an FDA audit, the reviewer has quick and convenient access to eDHRs in real time.”
Pushing the Limits
A constant challenge in EMS is meeting customer demands for miniaturization. A good example is reducing the size of power supplies to fit into smaller packages, without sacrificing power (and even increasing it). For example, MEAN WELL, a Taiwan-based manufacturer of standard power supplies for various industries, including the medical industry, recently released its MSP-1000 series, “which is a 1,000 W that is in the same enclosure as our previously released 600 W product,” stated production manager Kai Li. “We are also developing a 500 W open frame unit that is in a 5 x 3 inch standard footprint.”
Battery lifetime continues to be a significant challenge for wearable and implantable devices. Standard batteries are approaching their theoretical limits of power density—as a result, researchers are exploring new battery designs and materials. Scientists at MIT and the University of California-Berkeley have developed a lithium-ion battery that uses manganese as the cathode material instead of traditional cobalt or nickel, greatly boosting storage capacity and charging speed. A European research team recently produced a composite material of tin oxide nanoparticles enriched with antimony, attached to a base layer of graphene, which adds strength and conductivity to the material. Lithium-ion cells using this material for its electrodes can increase energy density by up to three times and greatly reduce charging time.
“Many OEMs are still surprised by how small and powerful drive solutions can be,” added Fritz. “Together with the high-efficiency operation and latest battery technologies, this allows for applications that were unthinkable in the past.”
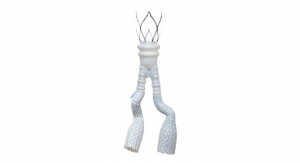
A good example is implantable smart pump systems that are used to treat various medical conditions. Sequana Medical has developed an active implant designed to treat ascites, a disorder in which large amounts of fluid accumulate in the peritoneal cavity. The implant system monitors the fluid accumulation and, when needed, pumps the fluid into the bladder of the patient. The lithium ion battery in the pump allows autonomous operation for several days and can be charged wirelessly through the skin of the patient. Motors provided by maxon precision motors keep the pump running smoothly. Being inside the body, the implant is completely sealed, including the complex electronics that control the motors and pump drive, evaluate the sensor signals, communicate with the charger, and manage battery life. To prevent clots from forming, the pump even performs a short movement without volume transport at regular intervals.
Ultimately, McClain believes the technology challenges that result from OEM demands will push the supply chain more than the EMS industry. At the EMS level, he noted, the challenge is simply to deliver an efficient, high-quality commercialization process, regardless of changes in inputs or market conditions. “To achieve this, we utilize real-time systems, robust process control, and strong focus on eliminating variation from the manufacturing process as primary tools. In terms of EMS evolution, I think it will be more along the lines of developing closer alignment with customer goals as a result of the ability to better integrate our systems.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.
A variety of evolving technology and business trends—including regulatory—are impacting the EMS landscape. For example, tax policy, tariffs, and new trade agreements are serious cost considerations MDMs must factor into medical device design and production. Regulatory requirements are also changing the relationships between MDMs, component suppliers, and service providers.
As a result, OEMs are re-evaluating their sourcing strategies and supply chain structure, looking for vertically integrated EMS suppliers that can help them reduce costs and navigate the complex and sometimes confusing regulatory processes. When they find the right partner, OEMs outsource their manufacturing and engineering needs to take advantage of the EMS vendor’s existing systems, facilities, and knowledge. Some EMS companies even provide complete global supply chain management and advanced quality systems, which may be more sophisticated than those of the OEM.
“The biggest trend we see is the need for EMS companies to be a valued business partner, as well as a manufacturing partner,” said Rick McClain, president of Milwaukee Electronics, a Glendale, Wis.-based provider of circuit board design and printed circuit board (PCB) assembly. “Tariffs and materials constraints are business challenges that require a team approach to address. EMS materials expertise, systems, facility options, and real-time communications capability are critical in this effort.”
What OEMs Want
OEMs are eager to design and manufacture smaller and smarter medical devices that incorporate Internet of Things (IoT) features. The capabilities of new sensor technologies continue to push the limits of design and manufacturing processes for electronics-embedded devices and equipment.
“OEMs are asking for more predictability, reliability, and flexibility out of their technologies, all while improving time to market and reducing cost,” said Lars Runquist, vice president of product development for Redgroup, a Minneapolis, Minn.-based contract manufacturer that develops and manufactures medical devices. “In response, contract manufacturers are becoming more integrated with production development, leveraging the latest technologies and skill sets.”
One way to keep costs down is by turning existing products into smart connected devices.
“There is a big demand for IoT solutions on devices that are already on the market, but have yet to be digitally connected,” said Walt Maclay, president of Voler Systems, a Sunnyvale, Calif.-based contract engineering firm that focuses on the design of wearable and IoT devices. “For example, orthopedic devices that have had no electronics can now be equipped with sensors and provide wireless communication. This can include information on the wear of joints, strain on the structure, or identification of the serial number using RFID [radio frequency identification].”
“Sometimes customers want to retrofit connectivity into an existing product, with the challenge of designing it in without affecting the patient’s familiarity with the current product and branding,” added Chris Conger, director of connected health technology for Phillips-Medisize, a Hudson, Wis.-based outsource provider of design development and manufacturing to the medical and pharmaceutical markets. “Others are looking for a new product or platform, often worn on the body, requiring thinner, flexible, smaller designs.”
OEMs expect medical EMS companies to deliver advanced medical manufacturing capabilities, including compliance management, automation, and data analytics. Increasingly, OEMs expect their EMS partners to solve manufacturing problems they cannot solve themselves—this is typically accomplished during the design for manufacturability (DFM) process. OEMs seek EMS services that can deliver mechatronic solutions that seamlessly integrate with the rest of the system. “These solutions include modified mechanical components, custom electronics design, special communication interfaces, and the manufacturing of the solution in compliance with the regulatory requirements,” said Christian Fritz, director of sales for motion control and electronics for maxon precision motors, a Fall River, Mass.-based provider of high-precision drive systems, including motor control electronics.
New Trends and Technologies
Flexible electronics is a segment of the market especially key for miniaturizing devices. They are essential for creating complex devices that can fit into small physical spaces, such as devices deployed within the human body. Many new flexible (and even stretchable) sensors are coming on the market, which can measure parameters such as stretching, angle, and force, all of which are important for flexible devices. Other flexible sensors can measure touch, compression, and pressure, while also detecting variances in performance.
Wearable medical devices, especially, are driving the need for flexible, smaller, lighter, and easier-to-use products. Key production technologies include miniaturized chip scale packaging, molded interconnect devices that integrate plastic and electronic components, and flexible electronics (copper and polyester films that have electronics printed on them with roll-to-roll processing). “Although these technologies require special equipment and manufacturing expertise, they give designers a wider window to deliver innovations that potentially improve patient comfort, compliance, and health,” said Conger.
In recent years, new processes have been developed that can manufacture flexible electronics in volume. “Some companies can make the integrated circuits or chips flexible by grinding them down to be very thin, and they can deposit flexible passive components—in fact, an entire electronic circuit can now be made flexible,” said Maclay.
Assembly technology, overall, has not seen major changes in the last few years; technological advancements are predominately in components and printed circuit board design. “From an EMS standpoint, the drivers of change would be the re-spin of ISO 13485, as well as several other quality systems, to drive additional focus on risk analysis and mitigation, a materials market with constraints that drive a need for better long-term planning, and tighter regulatory controls driving the need for more detailed quality data collection and device history record keeping,” said McClain.
Along these lines, more companies are investing in systems technologies that support real-time data collection and analysis while also improving communications with customers on issues related to material availability and product lifecycle planning. New developments in IoT, machine-to-machine communications, and the cloud (all of which depend upon electronics and sensors) have emerged that are helping solve complex manufacturing problems.
“These also provide more visibility across the global supply chain and enable quality teams to more quickly and efficiently ‘mistake proof’ production processes, so the most common causes of manufacturing error can be avoided,” said Charlie Mason, senior vice president of the medical market segment for Sanmina, a San Jose, Calif.-based provider of medical imaging systems and diagnostics equipment.
According to Susan Mucha, president of Powell-Mucha Consulting, an El Paso, Texas-based consulting firm that works with EMS providers, the biggest assembly challenge is material constraints. “Systems connectivity within the factory seems to be improving, but when you have a supply chain where confirmed orders can be cancelled the day they are scheduled to ship, the best systems in the world don’t change that,” she said. “Old-fashioned human intervention is often the only way to fix it. If the issues creating shortages in the components market can be resolved, [i.e., industry consolidation that cuts capacity, unanticipated demands for passives and some memory types, longer lead times], I think we will see a big increase in efficiency in the EMS industry, especially in smaller EMS companies.”
Electronic components can now be 3D-printed, which provides potential for shortening the product development times for electronics—a key step toward reducing the bottleneck in product development and keeping electronic development in pace with mechanical development. “We can design, print, and test a mechanical component multiple times in the same day with existing rapid prototyping technologies, including 3D printing,” said Runquist. “If electronics can continue to improve towards those timelines, we can validate our final products faster and get to market sooner.”
One of the most recent advances in 3D printing was accomplished by the University of Washington (UW) in collaboration with SIJ Technology and Japan’s National Institute of Advanced Industrial Science and Technology. UW purchased and installed an ultra-high-resolution electronics printer and integrated it with a roll-to-roll printer, making it the first system capable of high-throughput printing at sub-micron feature sizes. The printer will be available for academic and industry research and development, prototyping, and commercial manufacturing.
This system will be especially helpful in developing 3D-printed next-generation electronics that are sustainably manufactured with earth-abundant materials. Electronic devices such as touchscreens and other displays typically depend on rare earth and scarce materials that are transparent and electrically conductive, like indium tin oxide (ITO). According to UW, modeling has shown that electrodes made of earth-abundant materials (and therefore cheaper) can be patterned with micron-scale features, making them competitive with ITO electrodes. Copper-based transparent electrodes with nanoscale features, for example, could actually match or exceed the conductivity and transparency of conventional ITO electrodes.
“Our users will be able to print electronics using sustainable materials with finer control than ever before, and it will directly enable UW and industrial researchers to develop a sustainable alternative for a crucial element of flexible thin-film solar cells, displays, and touch screens,” said J. Devin MacKenzie, a University of Washington materials science mechanical engineering associate professor. “This printer, the first of its kind in the world, can also be used to make improved sensors and higher power batteries.”
Internet of Things
OEMs are constantly pushing for connectivity. EMS companies are being approached by customers who are eager to leverage IoT technologies (e.g., communication interfaces, security, and increased data collection and analytics) to broaden their services offerings in hot fields such as wearables. Major challenges, however, are security and data protection. The immediate and overwhelming demands by OEMs to build communications capabilities into so many devices creates intense design and production pressures on their EMS partners, who may not be experts with some of the communications technologies involved.
“There is a big push for IoT and tying products to customer’s cell phones,” said Thomas Allen, vice president of sales and marketing for TRICOR Systems, an Elgin, Ill.-based electronic contract manufacturer for the medical device industry. “While this seems like a good idea, it can add additional costs, risk, and security concerns. One example of these costs is that an OEM’s phone application software will have to support and keep current with multiple operating systems.”
In terms of operations, IoT and the cloud have an increasing impact on the factory floor and the entire supply chain. For example, five years ago, Sanmina realized that deploying its manufacturing execution system (MES) in the cloud to connect 60 factories and 25,000 pieces of manufacturing equipment had the potential to provide real-time supply chain visibility in ways not possible before. It created a “digital twin” of each factory in the cloud, with systems to collect and analyze critical manufacturing data. The connectivity of physical factories to a cloud platform enables real-time global visibility of manufacturing, as well as the ability to alert operators and engineers when human intervention is required. It also provides a mechanism for the management of supply chains across organizational boundaries.
“This technology provides real value to a chief operating officer having responsibility for 20 global factories,” said Mason. “When traveling, he or she can receive real-time notifications of problems in any of these factories, based on pre-programmed alert levels, or for products related to a particular customer. This same technology also provides real-time global supply chain visibility to a chief product officer.”
Regulatory Landscape
Regulatory requirements continue to change. This is especially true for devices deployed into the European market, where the new Medical Devices Regulation (MDR) has added many changes, including efficacy as a requirement. Most new devices must comply with the new regulations if they are placed on the market after May 2020 and existing devices must meet the new regulations after May 2025. Although this makes the regulations in Europe and the United States more harmonized, it also makes Europe a less desirable place to launch new products.
With more wireless and wired communication features being added to medical electronics devices, and increased scrutiny by regulatory bodies, “there is a now more of a ‘show me’ factor in process development,” said McClain. “Customers don’t just want to hear the process is good. They want to see a statistical sampling plan that proves the process is good. This is a result of regulatory bodies asking them for similar data.”
FDA quality requirements continue to evolve. For some products, the FDA (or the OEM) requires the tracking of every component, every piece of manufacturing equipment, and all operator activity related to the creation of a medical device throughout the manufacturing process. Therefore, EMS providers need to document production at a much higher level than they did just a few years ago. For example, when the FDA audits a manufacturing facility, it is critical for that company to produce correct, clear, and verifiable quality records in a timely manner. This is best accomplished using electronic acquisition and storage of records.
Sanmina has applied cloud and other technologies to create what it calls a medical “forced quality framework.” Equipment and material as well as production operators are connected via bar code scans to a single database in the cloud. If a component is scanned prior to assembly and is the wrong part or the wrong revision, the system will prevent it from being used. If an operator is not trained according to a recent change in work instructions, he or she will be prevented from logging onto that workstation and performing the activity until properly trained. If an assembly somehow arrives at the wrong step on the production line, it will automatically be rejected.
“A complete record of all operator activity, tests, process data, and component traceability data exists in this cloud database and is linked to electronic device history records [eDHR] for comprehensive digital record keeping,” said Mason. “If a production error is found down the road, the database can be easily searched to isolate production issues, minimizing the unnecessary recall of good products. During an FDA audit, the reviewer has quick and convenient access to eDHRs in real time.”
Pushing the Limits
A constant challenge in EMS is meeting customer demands for miniaturization. A good example is reducing the size of power supplies to fit into smaller packages, without sacrificing power (and even increasing it). For example, MEAN WELL, a Taiwan-based manufacturer of standard power supplies for various industries, including the medical industry, recently released its MSP-1000 series, “which is a 1,000 W that is in the same enclosure as our previously released 600 W product,” stated production manager Kai Li. “We are also developing a 500 W open frame unit that is in a 5 x 3 inch standard footprint.”
Battery lifetime continues to be a significant challenge for wearable and implantable devices. Standard batteries are approaching their theoretical limits of power density—as a result, researchers are exploring new battery designs and materials. Scientists at MIT and the University of California-Berkeley have developed a lithium-ion battery that uses manganese as the cathode material instead of traditional cobalt or nickel, greatly boosting storage capacity and charging speed. A European research team recently produced a composite material of tin oxide nanoparticles enriched with antimony, attached to a base layer of graphene, which adds strength and conductivity to the material. Lithium-ion cells using this material for its electrodes can increase energy density by up to three times and greatly reduce charging time.
“Many OEMs are still surprised by how small and powerful drive solutions can be,” added Fritz. “Together with the high-efficiency operation and latest battery technologies, this allows for applications that were unthinkable in the past.”
A good example is implantable smart pump systems that are used to treat various medical conditions. Sequana Medical has developed an active implant designed to treat ascites, a disorder in which large amounts of fluid accumulate in the peritoneal cavity. The implant system monitors the fluid accumulation and, when needed, pumps the fluid into the bladder of the patient. The lithium ion battery in the pump allows autonomous operation for several days and can be charged wirelessly through the skin of the patient. Motors provided by maxon precision motors keep the pump running smoothly. Being inside the body, the implant is completely sealed, including the complex electronics that control the motors and pump drive, evaluate the sensor signals, communicate with the charger, and manage battery life. To prevent clots from forming, the pump even performs a short movement without volume transport at regular intervals.
Ultimately, McClain believes the technology challenges that result from OEM demands will push the supply chain more than the EMS industry. At the EMS level, he noted, the challenge is simply to deliver an efficient, high-quality commercialization process, regardless of changes in inputs or market conditions. “To achieve this, we utilize real-time systems, robust process control, and strong focus on eliminating variation from the manufacturing process as primary tools. In terms of EMS evolution, I think it will be more along the lines of developing closer alignment with customer goals as a result of the ability to better integrate our systems.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.