Mark Crawford, Contributing Writer09.07.18
Market demands are pressuring medical device manufacturers (MDMs) to increase complexity and functionality, miniaturize, and meet tighter time-to-market requirements. Although they prefer to use proven and tested materials and designs, MDMs also realize they must adopt new and/or unfamiliar technologies to stay competitive—without compromising product quality.
Another pressure comes from regulatory agencies around the world, which are establishing more stringent rules for documenting development processes, including electronics (for example, EU MDR and IEC 60601-1-2 4th Edition for electromechanical devices). Global supply chain irregularities for key sub-components such as high-energy capacitors can also be a problem.
Insurance coverage is yet another driver in the medical device market, which can impact innovation and development costs. MDMs seek unit price reductions on component systems in an effort to offset shrinking insurance coverage—thereby creating more pressure on their contract manufacturers (CMs) to automate and invest in new architectures to support improved production capabilities.
These combined cost demands slow the pace of innovation. MDMs strive for the best possible solution at the lowest possible cost. They prefer standard, off-the-shelf equipment, parts, and materials whenever possible, but are willing to innovate when needed.
“For example, serial connection systems continue to be the standard,” said Mark Russell, senior global market manager of medical electronics for Bal Seal Engineering, a Foothill Ranch, Calif.-based manufacturer of custom electrical contact solutions for active medical implants. “System requirements for more connectivity, higher electrical performance, and smaller devices, however, are pushing the limits of many of the components used in implantable devices.”
To meet these needs, MDMs and CMs continue to advance their electronic capabilities. Wearables, in particular, is a fast-growing segment that requires improved electronic functionality—for example, flexible and stretchable substrates and more integrated electronics. MDMs expect new electronic components to support a wide range of output signals, consume minimal power, fit in small compact spaces, provide error checking, and offer exceptional reliability.
Ultimately, MDMs prefer to work with supply chain partners that provide multiple services, thereby shortening the supply chain and speeding up time to market. When they outsource electronics work, MDMs want a highly qualified part provider that is also a trusted partner they can rely on for product design input and regulatory advice. In order to be in high demand, electronic component providers must have the ability to innovate and prove to MDMs they can add value to complex designs (but also keep it simple when they can), push the limits of technology, and be an expert adviser on technology choices and regulations.
What OEMs Want
OEM demands are basic—higher functionality at lower costs. Integration of more components into smaller spaces is always in high demand. Hybrid connectors that reduce the number of interconnects by combining electrical with fiber, air, or fluid connections save time and money. Low power consumption is another popular request. Whenever possible, OEMs prefer to use off-the-shelf components, or a combination of off-the-shelf materials and custom assemblies. Custom work is more time-consuming and more expensive, so OEMs rarely choose the custom route unless it involves very large quantities. Using well-tested and readily available components not only speeds time to market, but also simplifies the bill of material by reducing investment in non-differentiating features and capabilities. The costs for validation, production tests, and sustaining engineering for custom products are often underestimated. In addition, much of the investment required for a fully custom design comes early in the process, when market potential and acceptance are only vaguely outlined, adding more risk to the project.
Reliability is another reason OEMs prefer to use established materials and components. Often the applications are truly critical, so manufacturers are reluctant to take any chances on unproven technologies. They also don’t want to re-invent the wheel, so designing with materials and components that have proven reliability in the industry is a cost and time advantage. For example, using contact systems that use established implantable materials reduces risk and helps satisfy requirements for FDA approval.
However, the increased willingness to adopt off-the-shelf components does not mean that OEMs are willing or able to sacrifice performance. “Today, much of the functionality of electronics systems is defined by software,” said Christian Fritz, director of sales for motion control and electronics for maxon precision motors, a Fall River, Mass.-based provider of high-precision drive technology, including motion control electronics, servo amplifiers, DC motors, and motion control sensors. “This provides the flexibility needed to modify off-the-shelf hardware that meets customer specific requirements. Customization can range from proprietary communication interfaces all the way to custom control intellectual property or application-specific state machines.”
When an MDM does undertake a contract custom project, the goal is usually to add more features and reduce size. Small components that offer very efficient performance are ideal; the less space the connections occupy, the more space is available for other functional requirements, or further size reduction.
“For example,” said Thomas Moore, technical support engineer for FUTEK Advanced Sensor Technology, an Irvine, Calif.-based manufacturer of test and measurement tools for the medical device industry, especially miniaturized sensor solutions, “the embedded solutions we design for OEMs are now required to store programmable calibration data, have low power consumption, a small footprint, and have multiple analog and digital signal output types, all in one package.”
Current Industry Trends
Requests for miniaturization and enhanced functionality continue to be the big drivers of innovation.
“Clients are asking for devices to be smaller and more user friendly with intuitive interfaces,” said Matt Valego, VP of sales and marketing for RBC Medical Innovations, a Lenexa, Kan.-based contract manufacturer of medical devices. “Additionally, wireless data communication and remote image-guided therapeutics have been a key component for several of our more recent initiatives.”
Julie Carlson, marketing manager for LEMO USA, a Rohnert Park, Calif.-based manufacturer of precision connectors, cables, and cable assemblies, has seen an increase in demand for smaller, high-density disposable connector solutions. The need for more data points requires more electrical contacts. “High density can be a bit more difficult when making the actual connector and contacts,” she said. “It takes much more advanced machinery to maintain higher tolerances to allow for precision mating and de-mating. On the assembly side, it requires more labor and skill, so the overall cost of the assembly increases.”
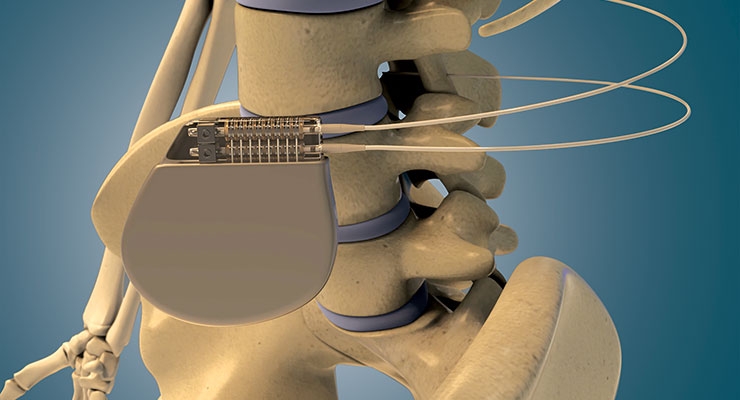
“Trends toward smaller contacts to support device size reduction are significant now,” added Russell. “For example, manufacturers of devices for various therapies are targeting up to a 50 percent reduction in size. For reference, a typical device used in spinal cord stimulation (SCS) is around 41cc. Also, typical lead systems have male leads around 1.3 mm—emerging therapies are aiming for leads as small as 0.9 mm, with some as small as 0.7 mm. This pushes the limits of allowable insertion forces due to limited lead rigidity.”
Another trend is the use of embedded active electronics to add intelligence into accessories. This is prevalent in many reusable, disposable, and “limited reuse” products. Embedding an ID chip into a cable assembly can provide intelligence to the accessory that will provide everything from operational settings to device limits for a given procedure and/or product. “For limited-reuse products, this ID chip can be used to record the number of times a device is used, as well as end usage when the maximum number of uses has been attained,” indicated David Cianciolo, director of engineering for Fischer Connectors, an Alpharetta, Ga.-based provider of high-performance connectors and cable assembly solutions for the medical industry.
MDMs can focus on developing core competencies when they outsource electronic applications. This helps them quickly iterate new designs, especially during the early design phases, and develop products with an optimized and balanced cost structure. “A growing number of electronics providers, often focused on special application tasks, support the device manufacturers not only with technology, but also valuable design expertise, customer specific modifications, and knowledge on regulatory rules and design practices,” said Fritz.
New Technologies and Methods
With a focus on technology innovations such as miniaturization, high-performance computing, and data analytics, MDMs are moving toward the use of highly complex electronic components for mission-critical tasks in medical applications. Advances in integration are improving system design. Improved header molding, batteries, advanced materials, and application-specific integrated circuits (ASIC) are driving device improvement in function and reliability. Wireless power transfer is being incorporated into more therapeutic options, such as external stimulation, implanted micro-stimulators, and fully functioning self-powered micro implants. Electronic improvements are also being made in deep ultraviolet light-emitting diodes (UVC LEDs), which are semiconductors that sterilize products with ultraviolet (UV) light.
“Deep ultraviolet LEDs offer integrated, portable, trackable UV disinfection for the smallest of devices,” said Rajul Randive, director of application development for Crystal IS Inc., a Green Island, N.Y.-based manufacturer of high-performance LEDs that emit UVC light for disinfecting water and surfaces, including medical devices. “We continue to reduce the size and power consumption while increasing the intensity of the UV energy. This allows for small, portable devices that offer quantifiable disinfection at the point-of-care—whether it’s at the hospital, clinic, or home.”
The Internet of Things (IoT) and big data are making their way into electronics manufacturing. Automation, networking, cloud computing, signal processing, and data analytics are core IoT technologies that open up design options and drive real-time decision making. Analysis of big data gathered by sensors embedded in equipment has the potential to maximize production efficiency and quality, while drastically reducing cost. Automation of manufacturing methods contributes to increased repeatability that can translate to better systems and cost savings.
“One of the biggest impacts of IoT will be on the standards used to communicate with custom electronics,” said Moore. “Manufacturers of these devices are specifying that custom components communicate as directly with the microprocessor as possible. This has made communication standards like serial peripheral interface, universal asynchronous receiver-transmitter, transistor-transistor logic, universal serial bus, or Bluetooth a requirement.”
Technologies that enable IoT are definitely of interest to the medical device industry, but will not be widely embraced until they are validated and considered safe, especially from the regulatory perspective. “The industry is also very aware that any development needs to take security and data protection into consideration,” cautioned Fritz. “Depending on the type and class of medical device, the adoption of IoT capabilities will vary greatly.”
Additive manufacturing and 3D printing, of course, are already being used in medical device manufacturing and have an impact on electronic component manufacturing. 3D printers continue to get faster as costs decrease; they use a wider variety of exotic materials, including flexible materials such as carbon fiber and Kevlar. Although 3D printing is still mostly used for prototypes, in some cases it can make production-ready parts. In terms of production, some factories already use 3D printing to manufacture assembly fixtures or tools that speed up machining or assembly. Electronics themselves have even been 3D-printed in lab settings, with good potential for commercial scale-up in the future.
Flexible, Stretchable, Wearable
Wearables are a hot market in the healthcare field; innovation is driven by consumer demands for more functionality, comfort, flexibility, and stretchability. This gives engineers more latitude for concepts, designs, and applications for energy storage devices and electronics. Such systems must accommodate large strain deformations, including bending, twisting, stretching, and compressing without breaking the delicate components and connections. Creating stretchable conductors involves either the implementation of strain reliefs through precise geometric patterning, the dispersion of stiff conductive filler in an elastomeric matrix, or using intrinsically stretchable conductive materials. “Patterned copper foils that are embedded in elastomeric sheets, which are closest to conventional electronic circuits processing, make up one end of the spectrum,” the National Institutes of Health stated in a recent publication. “Furthest from industry are the more recent circuits based on intrinsically stretchable liquid metals. These show extremely promising results; however, as a technology, liquid metal is not mature enough to be adapted.”
Considerable R&D is being spent on these advanced materials, especially liquid metals. In June 2018, Chinese scientists developed a hybrid material made from elastic polymer and liquid metal that can bend and stretch, offering potential for wearable circuits and biocompatible electronics. “These are the first flexible electronics that are at once highly conductive and stretchable, fully biocompatible, and able to be fabricated conveniently across size scales with micro-feature precision,” said senior researcher Xingyu Jiang, a professor at the National Center for Nanoscience and Technology in China. “We believe that they will have broad applications for both wearable electronics and implantable devices.”
Researchers at North Carolina State University have developed a printing method that allows them to print electronic circuits on flexible, stretchable substrates using silver nanowires. Silver nanowires have been recognized for their conductivity, flexibility, and stretchability, making them candidates for flexible circuits for a variety of potential applications, including wearables and prosthetic devices. “Our approach uses electrohydrodynamic printing, which relies on electrostatic force to eject the ink from the nozzle and draw it to the appropriate site on the substrate,” stated Jingyan Dong, a member of the research team and an associate professor at North Carolina State University. “This approach allows us to use a very wide nozzle, which prevents clogging, while still retaining very fine printing resolution.”
In October 2017, an engineering team from the U.S. Air Force Research Laboratory collaborated with Harvard University researchers to develop a new way to print stretchable, flexible electronics. Their method—called “hybrid 3D printing”—combines soft, conductive inks with a substrate to create stretchable, wearable electronic devices. Microcontroller chips and LEDs are embedded in a flexible, silver-infused thermoplastic polyurethane substrate. When tested, the hybrid-electronic devices functioned even after being stretched by more than 30 percent from the original size. “This is the first time a 3D printer has printed, in a single process, stretchable sensors with integrated microelectronic components,” said Dan Berrigan, a research scientist at the Air Force Research Lab. “Starting from nothing, the printer builds an entire stretchable circuit that blends the mechanical durability of printed components with the robust performance of off-the-shelf electronics.”
To create a highly sensitive artificial “skin,” University of Stanford engineers combined layers of advanced polymers and intricately patterned electronic meshes to provide the material’s elasticity and conductivity. Other layers serve as insulators to isolate the electronically sensitive materials. A two-inch square of this material contains about 6,000 individual signal-processing devices that act like synthetic nerve endings. The prototype can be stretched to double its original dimensions—and back again—and still maintain its ability to conduct electricity without cracks, delamination, or wrinkles. The researchers hope their work will lead to commercial production of sheets of polymer-based electronics embedded with a broad variety of sensors for a range of applications, including improving prosthetics or making direct connections with an individual’s nervous system.
Future Possibilities
Future trends in electronics will likely continue to be miniaturization and enhanced functionality—smaller devices that do more.
For example, “our goal is to build disconnection capabilities that produce the smallest footprint in the industry and support connection arrays up to 150 contacts,” said Russell. “At that number of connections, traditional system architecture becomes prohibitive—the surgeon’s options to implant the device will be limited by its size. We are developing new ways to use proven contact technology to meet these challenges.”
Research into new materials will continue. Graphene—a highly conductive and strong material—is gaining interest as a metal replacement. Graphene’s biocompatibility, coupled with its mechanical strength, is beneficial for various composite biomaterial applications. “In fact,” stated Roni Peleg, senior editor for Graphene Info, “researchers at the Michigan Technological University are gaining progress in 3D-printing replacement nerves and 3D bioprinting techniques. The team has developed polymer materials that can act as a scaffold for growing tissues and is working on integrating graphene as the electrical conductor.”
In addition, she noted, graphene-oxide (an oxygenated form of graphene) can bind to specific toxins to produce an enhanced signal that enables hypersensitive sensors that detect toxins at levels about 10 times lower than conventional sensors. “Another example is a sensor that predicts heart attacks, using graphene-oxide’s ability to detect specific microparticles in the blood that are released prior to heart attacks,” she added. “Various similar sensors are currently under research and development for the detection of a multitude of diseases, toxins, and biomarkers.”
Finally, a word of caution regarding this bright future, especially as electronic components become more complex and mission-critical: Counterfeit parts and products are a significant problem for the electronics industry. Some medical companies want better pricing to keep costs down and will seek out lower-cost products, which are often counterfeited “copy cats” that tend to be defective or have electrical issues and can cause risk to patient safety.
“Many times, these providers disguise themselves,” said Carlson. “They may have a website that looks like they are a USA company but, behind the scenes, the products are coming from China, mostly.”
Knock-offs of electronic components, such as connectors, can cause serious issues for purchasers. These parts are often deliberately designed to superficially resemble or imitate premium parts that have been manufactured with more stringent design specifications and that offer greater ultimate performance. This means that unwittingly using them as a component in an otherwise quality product could lead to accelerated equipment failure or malfunction, or to deficits in the performance of the particular end product.
Even more concerning is when these components find their way into medical devices, aerospace, and military applications, or into similar equipment or devices, “they may have life or death implications,” said Carlson.
Currently, electronics industry associations tend to focus limited resources primarily on more commonly pirated components such as chips for computers and smartphones. So far, there are no formal partnerships among connector manufacturers to combat counterfeit and copying issues, although informal discussions at trade shows and similar venues are not uncommon. “Therefore,” said Carlson, “each connector manufacturer must do what they can to protect their customers, end users, and their own reputations and products.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.
Another pressure comes from regulatory agencies around the world, which are establishing more stringent rules for documenting development processes, including electronics (for example, EU MDR and IEC 60601-1-2 4th Edition for electromechanical devices). Global supply chain irregularities for key sub-components such as high-energy capacitors can also be a problem.
Insurance coverage is yet another driver in the medical device market, which can impact innovation and development costs. MDMs seek unit price reductions on component systems in an effort to offset shrinking insurance coverage—thereby creating more pressure on their contract manufacturers (CMs) to automate and invest in new architectures to support improved production capabilities.
These combined cost demands slow the pace of innovation. MDMs strive for the best possible solution at the lowest possible cost. They prefer standard, off-the-shelf equipment, parts, and materials whenever possible, but are willing to innovate when needed.
“For example, serial connection systems continue to be the standard,” said Mark Russell, senior global market manager of medical electronics for Bal Seal Engineering, a Foothill Ranch, Calif.-based manufacturer of custom electrical contact solutions for active medical implants. “System requirements for more connectivity, higher electrical performance, and smaller devices, however, are pushing the limits of many of the components used in implantable devices.”
To meet these needs, MDMs and CMs continue to advance their electronic capabilities. Wearables, in particular, is a fast-growing segment that requires improved electronic functionality—for example, flexible and stretchable substrates and more integrated electronics. MDMs expect new electronic components to support a wide range of output signals, consume minimal power, fit in small compact spaces, provide error checking, and offer exceptional reliability.
Ultimately, MDMs prefer to work with supply chain partners that provide multiple services, thereby shortening the supply chain and speeding up time to market. When they outsource electronics work, MDMs want a highly qualified part provider that is also a trusted partner they can rely on for product design input and regulatory advice. In order to be in high demand, electronic component providers must have the ability to innovate and prove to MDMs they can add value to complex designs (but also keep it simple when they can), push the limits of technology, and be an expert adviser on technology choices and regulations.
What OEMs Want
OEM demands are basic—higher functionality at lower costs. Integration of more components into smaller spaces is always in high demand. Hybrid connectors that reduce the number of interconnects by combining electrical with fiber, air, or fluid connections save time and money. Low power consumption is another popular request. Whenever possible, OEMs prefer to use off-the-shelf components, or a combination of off-the-shelf materials and custom assemblies. Custom work is more time-consuming and more expensive, so OEMs rarely choose the custom route unless it involves very large quantities. Using well-tested and readily available components not only speeds time to market, but also simplifies the bill of material by reducing investment in non-differentiating features and capabilities. The costs for validation, production tests, and sustaining engineering for custom products are often underestimated. In addition, much of the investment required for a fully custom design comes early in the process, when market potential and acceptance are only vaguely outlined, adding more risk to the project.
Reliability is another reason OEMs prefer to use established materials and components. Often the applications are truly critical, so manufacturers are reluctant to take any chances on unproven technologies. They also don’t want to re-invent the wheel, so designing with materials and components that have proven reliability in the industry is a cost and time advantage. For example, using contact systems that use established implantable materials reduces risk and helps satisfy requirements for FDA approval.
However, the increased willingness to adopt off-the-shelf components does not mean that OEMs are willing or able to sacrifice performance. “Today, much of the functionality of electronics systems is defined by software,” said Christian Fritz, director of sales for motion control and electronics for maxon precision motors, a Fall River, Mass.-based provider of high-precision drive technology, including motion control electronics, servo amplifiers, DC motors, and motion control sensors. “This provides the flexibility needed to modify off-the-shelf hardware that meets customer specific requirements. Customization can range from proprietary communication interfaces all the way to custom control intellectual property or application-specific state machines.”
When an MDM does undertake a contract custom project, the goal is usually to add more features and reduce size. Small components that offer very efficient performance are ideal; the less space the connections occupy, the more space is available for other functional requirements, or further size reduction.
“For example,” said Thomas Moore, technical support engineer for FUTEK Advanced Sensor Technology, an Irvine, Calif.-based manufacturer of test and measurement tools for the medical device industry, especially miniaturized sensor solutions, “the embedded solutions we design for OEMs are now required to store programmable calibration data, have low power consumption, a small footprint, and have multiple analog and digital signal output types, all in one package.”
Current Industry Trends
Requests for miniaturization and enhanced functionality continue to be the big drivers of innovation.
“Clients are asking for devices to be smaller and more user friendly with intuitive interfaces,” said Matt Valego, VP of sales and marketing for RBC Medical Innovations, a Lenexa, Kan.-based contract manufacturer of medical devices. “Additionally, wireless data communication and remote image-guided therapeutics have been a key component for several of our more recent initiatives.”
Julie Carlson, marketing manager for LEMO USA, a Rohnert Park, Calif.-based manufacturer of precision connectors, cables, and cable assemblies, has seen an increase in demand for smaller, high-density disposable connector solutions. The need for more data points requires more electrical contacts. “High density can be a bit more difficult when making the actual connector and contacts,” she said. “It takes much more advanced machinery to maintain higher tolerances to allow for precision mating and de-mating. On the assembly side, it requires more labor and skill, so the overall cost of the assembly increases.”
“Trends toward smaller contacts to support device size reduction are significant now,” added Russell. “For example, manufacturers of devices for various therapies are targeting up to a 50 percent reduction in size. For reference, a typical device used in spinal cord stimulation (SCS) is around 41cc. Also, typical lead systems have male leads around 1.3 mm—emerging therapies are aiming for leads as small as 0.9 mm, with some as small as 0.7 mm. This pushes the limits of allowable insertion forces due to limited lead rigidity.”
Another trend is the use of embedded active electronics to add intelligence into accessories. This is prevalent in many reusable, disposable, and “limited reuse” products. Embedding an ID chip into a cable assembly can provide intelligence to the accessory that will provide everything from operational settings to device limits for a given procedure and/or product. “For limited-reuse products, this ID chip can be used to record the number of times a device is used, as well as end usage when the maximum number of uses has been attained,” indicated David Cianciolo, director of engineering for Fischer Connectors, an Alpharetta, Ga.-based provider of high-performance connectors and cable assembly solutions for the medical industry.
MDMs can focus on developing core competencies when they outsource electronic applications. This helps them quickly iterate new designs, especially during the early design phases, and develop products with an optimized and balanced cost structure. “A growing number of electronics providers, often focused on special application tasks, support the device manufacturers not only with technology, but also valuable design expertise, customer specific modifications, and knowledge on regulatory rules and design practices,” said Fritz.
New Technologies and Methods
With a focus on technology innovations such as miniaturization, high-performance computing, and data analytics, MDMs are moving toward the use of highly complex electronic components for mission-critical tasks in medical applications. Advances in integration are improving system design. Improved header molding, batteries, advanced materials, and application-specific integrated circuits (ASIC) are driving device improvement in function and reliability. Wireless power transfer is being incorporated into more therapeutic options, such as external stimulation, implanted micro-stimulators, and fully functioning self-powered micro implants. Electronic improvements are also being made in deep ultraviolet light-emitting diodes (UVC LEDs), which are semiconductors that sterilize products with ultraviolet (UV) light.
“Deep ultraviolet LEDs offer integrated, portable, trackable UV disinfection for the smallest of devices,” said Rajul Randive, director of application development for Crystal IS Inc., a Green Island, N.Y.-based manufacturer of high-performance LEDs that emit UVC light for disinfecting water and surfaces, including medical devices. “We continue to reduce the size and power consumption while increasing the intensity of the UV energy. This allows for small, portable devices that offer quantifiable disinfection at the point-of-care—whether it’s at the hospital, clinic, or home.”
The Internet of Things (IoT) and big data are making their way into electronics manufacturing. Automation, networking, cloud computing, signal processing, and data analytics are core IoT technologies that open up design options and drive real-time decision making. Analysis of big data gathered by sensors embedded in equipment has the potential to maximize production efficiency and quality, while drastically reducing cost. Automation of manufacturing methods contributes to increased repeatability that can translate to better systems and cost savings.
“One of the biggest impacts of IoT will be on the standards used to communicate with custom electronics,” said Moore. “Manufacturers of these devices are specifying that custom components communicate as directly with the microprocessor as possible. This has made communication standards like serial peripheral interface, universal asynchronous receiver-transmitter, transistor-transistor logic, universal serial bus, or Bluetooth a requirement.”
Technologies that enable IoT are definitely of interest to the medical device industry, but will not be widely embraced until they are validated and considered safe, especially from the regulatory perspective. “The industry is also very aware that any development needs to take security and data protection into consideration,” cautioned Fritz. “Depending on the type and class of medical device, the adoption of IoT capabilities will vary greatly.”
Additive manufacturing and 3D printing, of course, are already being used in medical device manufacturing and have an impact on electronic component manufacturing. 3D printers continue to get faster as costs decrease; they use a wider variety of exotic materials, including flexible materials such as carbon fiber and Kevlar. Although 3D printing is still mostly used for prototypes, in some cases it can make production-ready parts. In terms of production, some factories already use 3D printing to manufacture assembly fixtures or tools that speed up machining or assembly. Electronics themselves have even been 3D-printed in lab settings, with good potential for commercial scale-up in the future.
Flexible, Stretchable, Wearable
Wearables are a hot market in the healthcare field; innovation is driven by consumer demands for more functionality, comfort, flexibility, and stretchability. This gives engineers more latitude for concepts, designs, and applications for energy storage devices and electronics. Such systems must accommodate large strain deformations, including bending, twisting, stretching, and compressing without breaking the delicate components and connections. Creating stretchable conductors involves either the implementation of strain reliefs through precise geometric patterning, the dispersion of stiff conductive filler in an elastomeric matrix, or using intrinsically stretchable conductive materials. “Patterned copper foils that are embedded in elastomeric sheets, which are closest to conventional electronic circuits processing, make up one end of the spectrum,” the National Institutes of Health stated in a recent publication. “Furthest from industry are the more recent circuits based on intrinsically stretchable liquid metals. These show extremely promising results; however, as a technology, liquid metal is not mature enough to be adapted.”
Considerable R&D is being spent on these advanced materials, especially liquid metals. In June 2018, Chinese scientists developed a hybrid material made from elastic polymer and liquid metal that can bend and stretch, offering potential for wearable circuits and biocompatible electronics. “These are the first flexible electronics that are at once highly conductive and stretchable, fully biocompatible, and able to be fabricated conveniently across size scales with micro-feature precision,” said senior researcher Xingyu Jiang, a professor at the National Center for Nanoscience and Technology in China. “We believe that they will have broad applications for both wearable electronics and implantable devices.”
Researchers at North Carolina State University have developed a printing method that allows them to print electronic circuits on flexible, stretchable substrates using silver nanowires. Silver nanowires have been recognized for their conductivity, flexibility, and stretchability, making them candidates for flexible circuits for a variety of potential applications, including wearables and prosthetic devices. “Our approach uses electrohydrodynamic printing, which relies on electrostatic force to eject the ink from the nozzle and draw it to the appropriate site on the substrate,” stated Jingyan Dong, a member of the research team and an associate professor at North Carolina State University. “This approach allows us to use a very wide nozzle, which prevents clogging, while still retaining very fine printing resolution.”
In October 2017, an engineering team from the U.S. Air Force Research Laboratory collaborated with Harvard University researchers to develop a new way to print stretchable, flexible electronics. Their method—called “hybrid 3D printing”—combines soft, conductive inks with a substrate to create stretchable, wearable electronic devices. Microcontroller chips and LEDs are embedded in a flexible, silver-infused thermoplastic polyurethane substrate. When tested, the hybrid-electronic devices functioned even after being stretched by more than 30 percent from the original size. “This is the first time a 3D printer has printed, in a single process, stretchable sensors with integrated microelectronic components,” said Dan Berrigan, a research scientist at the Air Force Research Lab. “Starting from nothing, the printer builds an entire stretchable circuit that blends the mechanical durability of printed components with the robust performance of off-the-shelf electronics.”
To create a highly sensitive artificial “skin,” University of Stanford engineers combined layers of advanced polymers and intricately patterned electronic meshes to provide the material’s elasticity and conductivity. Other layers serve as insulators to isolate the electronically sensitive materials. A two-inch square of this material contains about 6,000 individual signal-processing devices that act like synthetic nerve endings. The prototype can be stretched to double its original dimensions—and back again—and still maintain its ability to conduct electricity without cracks, delamination, or wrinkles. The researchers hope their work will lead to commercial production of sheets of polymer-based electronics embedded with a broad variety of sensors for a range of applications, including improving prosthetics or making direct connections with an individual’s nervous system.
Future Possibilities
Future trends in electronics will likely continue to be miniaturization and enhanced functionality—smaller devices that do more.
For example, “our goal is to build disconnection capabilities that produce the smallest footprint in the industry and support connection arrays up to 150 contacts,” said Russell. “At that number of connections, traditional system architecture becomes prohibitive—the surgeon’s options to implant the device will be limited by its size. We are developing new ways to use proven contact technology to meet these challenges.”
Research into new materials will continue. Graphene—a highly conductive and strong material—is gaining interest as a metal replacement. Graphene’s biocompatibility, coupled with its mechanical strength, is beneficial for various composite biomaterial applications. “In fact,” stated Roni Peleg, senior editor for Graphene Info, “researchers at the Michigan Technological University are gaining progress in 3D-printing replacement nerves and 3D bioprinting techniques. The team has developed polymer materials that can act as a scaffold for growing tissues and is working on integrating graphene as the electrical conductor.”
In addition, she noted, graphene-oxide (an oxygenated form of graphene) can bind to specific toxins to produce an enhanced signal that enables hypersensitive sensors that detect toxins at levels about 10 times lower than conventional sensors. “Another example is a sensor that predicts heart attacks, using graphene-oxide’s ability to detect specific microparticles in the blood that are released prior to heart attacks,” she added. “Various similar sensors are currently under research and development for the detection of a multitude of diseases, toxins, and biomarkers.”
Finally, a word of caution regarding this bright future, especially as electronic components become more complex and mission-critical: Counterfeit parts and products are a significant problem for the electronics industry. Some medical companies want better pricing to keep costs down and will seek out lower-cost products, which are often counterfeited “copy cats” that tend to be defective or have electrical issues and can cause risk to patient safety.
“Many times, these providers disguise themselves,” said Carlson. “They may have a website that looks like they are a USA company but, behind the scenes, the products are coming from China, mostly.”
Knock-offs of electronic components, such as connectors, can cause serious issues for purchasers. These parts are often deliberately designed to superficially resemble or imitate premium parts that have been manufactured with more stringent design specifications and that offer greater ultimate performance. This means that unwittingly using them as a component in an otherwise quality product could lead to accelerated equipment failure or malfunction, or to deficits in the performance of the particular end product.
Even more concerning is when these components find their way into medical devices, aerospace, and military applications, or into similar equipment or devices, “they may have life or death implications,” said Carlson.
Currently, electronics industry associations tend to focus limited resources primarily on more commonly pirated components such as chips for computers and smartphones. So far, there are no formal partnerships among connector manufacturers to combat counterfeit and copying issues, although informal discussions at trade shows and similar venues are not uncommon. “Therefore,” said Carlson, “each connector manufacturer must do what they can to protect their customers, end users, and their own reputations and products.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.