Sudeep Kolluri, Solutioning Lead, Freyr06.04.18
The medical device market is one of the largest industries in the healthcare sector, which has seen significant growth in the last 15 years. Harnessing the benefits of the digital revolution and medical technology, the industry is in a state of continuous transformation. Mobile medical applications (MMA) represent one such transformative invention the industry has provided to healthcare professionals. An MMA is a piece of software that runs on a smartphone and/or other mobile communication device, transforming the mobile platform into a medical device to perform a specific healthcare function.
According to Research and Markets, the global market for mobile health applications is currently valued at approximately $28.32 billion and is expected to reach $102.35 billion by 2023. Complex data analytics and mobile technologies are enabling and driving the integration of mobile devices into the healthcare sector, which in turn are catering to the requirements of healthcare professionals, patients, and general consumers as well. They have also contributed to simpler remote monitoring; better communication; and care coordination among doctors, nurses, and other specialists involved in the diagnosis and treatment of diseases.
Depending upon the functionality, MMAs can be broadly segmented into one of several categories.
Chronic Care Management Apps: These include apps to manage blood pressure, cancer care, diabetes care, mental health, and other illnesses.
Medical Apps: These apps are mainly used by healthcare professionals. Examples include medical education apps; doctor consultation/appointment apps; patient management and monitoring, etc.; clinical decision support systems, which assist doctors/physicians in diagnosing various health conditions, are also categorized under medical apps.
General Health and Fitness Apps: These apps constitute almost 75 percent of MMAs found on app stores. These are related to nutrition, health tracking, fitness, and weight loss, along with wearable technology sensors and other health monitors.
Medication Management Apps: These apps help in keeping track of medicine intake to ensure proper dosing at required intervals.
Personal Health Record Apps: These applications allow patients to store their medical conditions data, history, allergies, etc.
Women’s Health Apps: This segment includes apps for pregnancy, fertility, breastfeeding, etc.
Regulatory Framework for Mobile Medical Applications
Currently, there are almost 170,000 MMAs available on various mobile platforms. Of these, only a small percentage (approximately 2-3 percent) resemble the definition of a medical device, thereby requiring approval by the respective Health Authority (HA) prior to public release. While the MMA market is undergoing rapid transformation, the lack of up-to-date and comprehensive regulatory guidance is creating a challenge for app developers throughout the world. Although major markets like the United States and European Union (EU) have published their regulations, there exists a gray area for mobile medical-oriented application developers. There is ambiguity regarding the conformity of these apps to the regulations. Compared with EU, the U.S. Food and Drug Administration’s (FDA) regulations are more detailed and provide better clarification on the criteria for the MMAs to qualify as a medical device.
U.S. MMA Regulations
In September 2015, FDA released a guidance with the name “Mobile Medical Applications,” which superseded the February 2015 version. As per the FDA’s guidance, MMA is defined as:
“…mobile app that meets the definition of device in section 201(h) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and either is intended to be used as an accessory to a regulated medical device or to transform a mobile platform into a regulated medical device.”
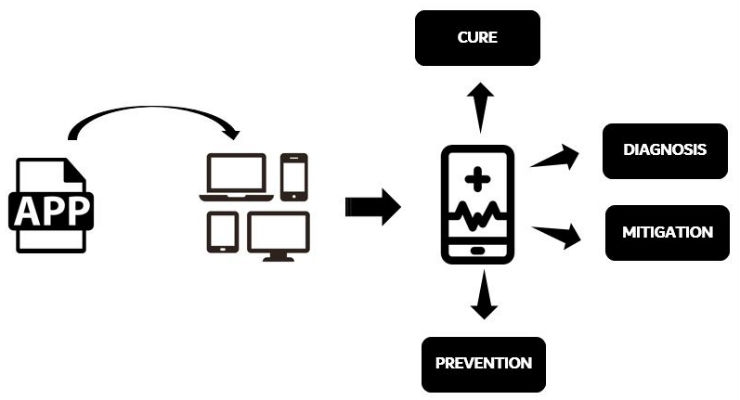
Regardless of the mobile device hardware or platform, consideration of MMA as a medical device is determined by the intended use of the mobile app to cure, prevent, mitigate, and treat a disease. FDA determines the intended use through the examination of advertisements, labeling claims, and oral and written statements by the manufacturer.
FDA considers categorization of MMAs as follows:
EU MMA Regulations
The EU’s definition of a medical device is similar to the U.S.; the regulatory process applies only to those MMAs that meet the EU definition of a medical device. As per EU Medical Device Regulation, the definition of a medical device is:
“Medical device means any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:
Medical Apps: Generally used in prevention, diagnosis, and treatment of diseases (CE certification is required for these apps).
Non-Medical Apps: Related to fitness, lifestyle, and well-being.
At the national level, several EU member states such as France, Spain, Germany, and Italy are participating in developing the guidance for MMAs to provide clarification on the European Commission guidelines. To expand mobile capabilities in the healthcare sector, app developers in EU should also take into consideration the latest General Data Protection Regulations (GDPR), which were scheduled to come into effect on May 25. These regulations are expected to have several implications on personal data security and data privacy. They also require all app developers processing users’ personal data—either EU-based or not—to strictly adhere to the latest GDPR policy.
Conclusion
Regulatory authorities in the EU and U.S. are diligently working on creating overarching policies for MMAs. The ideology behind the regulations for MMAs in the EU and U.S. is almost similar, but the FDA is, by far, further along in developing guidance in a detailed manner. In this environment of technological transformations, it is crucial for HAs to maintain updated draft regulations that strike a balance toward ensuring safety and efficacy, while promoting innovation.
Sudeep Kolluri is a solutioning lead at Freyr, handling medical devices approvals for EU and LATAM. He has work experience across regulatory intelligence, design strategy for approval, and registration of the products in these regions for various categories of medical devices.
According to Research and Markets, the global market for mobile health applications is currently valued at approximately $28.32 billion and is expected to reach $102.35 billion by 2023. Complex data analytics and mobile technologies are enabling and driving the integration of mobile devices into the healthcare sector, which in turn are catering to the requirements of healthcare professionals, patients, and general consumers as well. They have also contributed to simpler remote monitoring; better communication; and care coordination among doctors, nurses, and other specialists involved in the diagnosis and treatment of diseases.
Depending upon the functionality, MMAs can be broadly segmented into one of several categories.
Chronic Care Management Apps: These include apps to manage blood pressure, cancer care, diabetes care, mental health, and other illnesses.
Medical Apps: These apps are mainly used by healthcare professionals. Examples include medical education apps; doctor consultation/appointment apps; patient management and monitoring, etc.; clinical decision support systems, which assist doctors/physicians in diagnosing various health conditions, are also categorized under medical apps.
General Health and Fitness Apps: These apps constitute almost 75 percent of MMAs found on app stores. These are related to nutrition, health tracking, fitness, and weight loss, along with wearable technology sensors and other health monitors.
Medication Management Apps: These apps help in keeping track of medicine intake to ensure proper dosing at required intervals.
Personal Health Record Apps: These applications allow patients to store their medical conditions data, history, allergies, etc.
Women’s Health Apps: This segment includes apps for pregnancy, fertility, breastfeeding, etc.
Regulatory Framework for Mobile Medical Applications
Currently, there are almost 170,000 MMAs available on various mobile platforms. Of these, only a small percentage (approximately 2-3 percent) resemble the definition of a medical device, thereby requiring approval by the respective Health Authority (HA) prior to public release. While the MMA market is undergoing rapid transformation, the lack of up-to-date and comprehensive regulatory guidance is creating a challenge for app developers throughout the world. Although major markets like the United States and European Union (EU) have published their regulations, there exists a gray area for mobile medical-oriented application developers. There is ambiguity regarding the conformity of these apps to the regulations. Compared with EU, the U.S. Food and Drug Administration’s (FDA) regulations are more detailed and provide better clarification on the criteria for the MMAs to qualify as a medical device.
U.S. MMA Regulations
In September 2015, FDA released a guidance with the name “Mobile Medical Applications,” which superseded the February 2015 version. As per the FDA’s guidance, MMA is defined as:
“…mobile app that meets the definition of device in section 201(h) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and either is intended to be used as an accessory to a regulated medical device or to transform a mobile platform into a regulated medical device.”
Regardless of the mobile device hardware or platform, consideration of MMA as a medical device is determined by the intended use of the mobile app to cure, prevent, mitigate, and treat a disease. FDA determines the intended use through the examination of advertisements, labeling claims, and oral and written statements by the manufacturer.
FDA considers categorization of MMAs as follows:
- Apps that do not meet the definition of a medical device as per the FD&C Act. The FD&C Act is the legal authority through which the FDA regulates medical devices; it contains provisions and regulatory requirements that apply to medical devices. FDA does not regulate mobile medical apps that do not qualify as medical devices. Examples: medical dictionaries and encyclopedias; educational apps for healthcare professionals and patients.
- Apps that may be medical devices as per the definition but require no regulation by the FDA on account of low potential risk. Most of the general health and wellness mobile applications; for instance, fitness and nutrition apps are subject to enforcement discretion. Examples: mobile apps that perform simple calculations routinely used in clinical practice such as body mass index and delivery date estimator; patient health record systems; medical device data systems (used to transfer, format, store, convert, and display medical device data).
- Apps that are subject to FDA oversight due to considerable risk to patient safety. Examples: apps that transform the mobile platform into a Class II regulated medical device by displaying radiological images for diagnosis; apps that require an attachment to the mobile device to measure blood glucose levels; mobile apps that wirelessly control computed tomography or X-ray machines.
- Software intended for administrative support of a healthcare facility
- Software promoting and maintaining healthy lifestyle
- Medical device data systems
- Electronic patient record software
- Some of the clinical decision support systems
| USFDA Regulations Applicable for MMAs and Software as Medical Device (SaMD) | ||
| Regulation | Description | |
| Final Regulations | Mobile Medical Applications (MMAs) | This guidance explains the medical device qualifying criteria for the mobile medical applications. |
| Software as Medical Device (SaMD) | An IMDRF (GHTF) document adopted by USFDA to define SaMD. | |
| SaMD Clinical Evaluation | An IMDRF guidance adopted by USFDA on the clinical evaluation of SaMD. | |
| General Principles of Software Validation | It explains how the medical device quality system applies to MMAs. | |
|
Off-The-Shelf Software Use in Medical Devices |
It details the documentation needed in the premarket submission for medical devices using Off-the-Shelf Software. | |
| MDDS Rule Federal Register Notice | The document clarifies MDDS classification rules. | |
| Applying Human Factors and Usability Engineering to Medical Devices | This guidance focusses on user interfaces, display, controls and instructions for use. | |
|
Clinical Performance Assessment: Considerations for Computer-Assisted Detection Devices Applied to Radiology Images and Radiology Device Data - Premarket Approval (PMA) and Premarket Notification [510(k)] Submissions |
This document provides guidance regarding clinical performance assessment studies for Computer Assisted Detection (CAD) devices applied to radiology images and radiology device data. | |
| Computer-Assisted Detection Devices Applied to Radiology Images and Radiology Device Data – Premarket Notification [510(k)] Submissions | The document is for premarket notification (510(k)) submissions for CAD devices applied to radiology images and radiology device data. | |
| Post market Management of Cybersecurity in Medical Devices | The guidance explains the post market cybersecurity measures to be followed for mobile medical applications. | |
| General Wellness: Policy for Low-Risk Devices | It defines the criteria for low-risk general wellness applications. | |
| 21st Century Cures Act | This document features the salient features and the implications of 21st Century Cures Act. | |
| Draft Regulations | Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act | This draft explains information on the effect of 21st Century Cures Act on MMAs and SaMD in general. |
| Clinical and Patient Decision Support Software | Contains information on why the medical device status is withdrawn for certain clinical decision support software, post the 21st Century Cures Act. | |
EU MMA Regulations
The EU’s definition of a medical device is similar to the U.S.; the regulatory process applies only to those MMAs that meet the EU definition of a medical device. As per EU Medical Device Regulation, the definition of a medical device is:
“Medical device means any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:
- Diagnosis, prevention, monitoring, treatment or alleviation of disease;
- Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap;
- Investigation, replacement or modification of the anatomy or of a physiological process;
- Control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.”
Medical Apps: Generally used in prevention, diagnosis, and treatment of diseases (CE certification is required for these apps).
Non-Medical Apps: Related to fitness, lifestyle, and well-being.
At the national level, several EU member states such as France, Spain, Germany, and Italy are participating in developing the guidance for MMAs to provide clarification on the European Commission guidelines. To expand mobile capabilities in the healthcare sector, app developers in EU should also take into consideration the latest General Data Protection Regulations (GDPR), which were scheduled to come into effect on May 25. These regulations are expected to have several implications on personal data security and data privacy. They also require all app developers processing users’ personal data—either EU-based or not—to strictly adhere to the latest GDPR policy.
Conclusion
Regulatory authorities in the EU and U.S. are diligently working on creating overarching policies for MMAs. The ideology behind the regulations for MMAs in the EU and U.S. is almost similar, but the FDA is, by far, further along in developing guidance in a detailed manner. In this environment of technological transformations, it is crucial for HAs to maintain updated draft regulations that strike a balance toward ensuring safety and efficacy, while promoting innovation.
Sudeep Kolluri is a solutioning lead at Freyr, handling medical devices approvals for EU and LATAM. He has work experience across regulatory intelligence, design strategy for approval, and registration of the products in these regions for various categories of medical devices.