Sam Brusco, Associate Editor06.04.18
Though merely 15 years ago, given the breakneck pace of innovation these days, 2003 seems a world away in terms of science and technology. It was the year the Concorde supersonic jet made its final flight; the year Kodak released the first digital camera with an organic light-emitting diode (OLED) display; and the year the National Human Genome Research Institute and the Department of Energy unveiled the successfully completed Human Genome Project, the culmination of a 13-year effort to sequence the three billion DNA letters in the human genome.
2003 also marked Medical Product Outsourcing’s flagship issue, featuring the very first Top 30 Global Medical Device Companies report (this year’s version to be featured next month—stay tuned).
That year’s list of the elite was championed by global medical device, drug, and consumer products company Johnson & Johnson, which would remain at the top spot for over a decade. J&J’s $11.6 billion in medical device revenue now seems paltry compared to 2017’s leader—Medtronic plc—which touted $28.8 billion in sales.
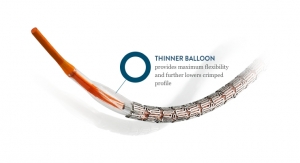
Partially responsible for J&J’s windfall in 2003 resulted from Cordis Corporation’s (then a J&J business, now owned by Cardinal Health) April launch of a landmark treatment for coronary artery disease, and the first commercial device of its type—the Cypher drug-eluting stent, or DES for short.
Cordis’ Cypher DES was hailed as the first combination drug-device intended to help reduce restenosis (blockage) of a coronary artery treated with a stent. Restenosis was then—and continues to be—an enormous challenge in long-term stent implantations used for interventional cardiology treatment. To prevent it, Cypher was coated in sirolimus, a macrolide compound that is also used to prevent organ transplant rejection and treat LAM—a rare, progressive, and systemic lung disease.
“Cordis is very pleased to bring this remarkable and innovative treatment to patients, hospitals, and interventional cardiologists,” said then Johnson & Johnson Company Group Chairman Robert Croce upon Cypher’s U.S. launch. “Clinical evidence and experience with more than 50,000 patients treated to date in nearly 60 countries suggests the Cypher Stent represents the beginning of a new era in interventional cardiology—an era in which the combination of drugs and devices substantially improves patient outcomes.”
DES technology was regarded to be such a significant improvement on current bare metal stent technology that the Centers for Medicare and Medicaid services (CMS) approved Cypher’s incremental reimbursement in August 2002, a full nine months before J&J launched Cypher in the U.S. The new policy provided significant incremental reimbursement over and above 2003’s bare metal stent reimbursement levels—undoubtedly due to Cypher’s demonstrated sustained reduction in blockage incidence by over 90 percent in Cordis’ landmark U.S. SIRIUS trial.
A number of other medical technology giants were racing to commercialize their own DES products that year as well—J&J was simply the first to reach the U.S. market, granting it a sizeable head start. Around the same time J&J was gearing up to launch Cypher, Boston Scientific Corp. had achieved CE mark approval for its Taxus paclitaxel-eluting coronary stent. Taxus launched in the U.S. in March 2004, almost a full year behind Cypher. Paclitaxel, Boston Scientific’s drug of choice for the Taxus DES, is also used as a chemotherapy medication to treat a number of types of cancer.
“The approval of the Taxus system marks an important opportunity for clinicians in the United States,” said Gregg Stone, M.D., then vice chairman of the Cardiovascular Research Foundation at the Lenox Hill Heart and Vascular Institute in New York, and principal investigator of the Taxus IV clinical trial. “In paclitaxel, we now have a multi-functional drug that is safe and highly effective. The Taxus system has shown impressive results across a wide range of patients. Its performance has been particularly impressive in challenging cases such as patients with diabetes, small vessels, and long lesions.”
Guidant Corporation—which Boston Scientific acquired in 2015, after settling a lawsuit with J&J over the deal—had its own stent in the ring around that time, this one coated in everolimus, a sirolimus derivative used to prevent organ transplant rejection and treat renal cell cancer. However, rather than bringing its product to market itself, in February 2004 Guidant began a deal with Cordis to co-promote Cypher and assist the company in developing a Cypher stent utilizing Guidant’s stent delivery system. The agreement granted Guidant immediate entry into the U.S. DES market and settled ongoing stent-related patent disputes between the companies.
Medtronic was also in the midst of clinical trials for its own Endeavor phosphorylcholine-coated DES in 2003, but did not achieve FDA approval and launch of the device until 2008, though it was the first DES approved in four years. Phosphorylcholine, unlike the other drug compounds used previously, is exclusively meant to prevent coronary artery blockage.
Today, a majority of stents used in interventional cardiology procedures are coated in some antiproliferative pharmaceutical agent to prevent restenosis. J&J exited the DES market in 2011 due to safety concerns and fierce competition from rival products, but the market remains strong—according to Grand View Research, it was valued at $5.6 billion in 2015 with major players Boston Scientific, Medtronic, Abbott Laboratories, Biosensors International, Biotronik, Lepu Medical Technology, Terumo Medical, Cook Medical, Shandong JW Medical Systems, and Stentys leading the charge.
2003 also marked Medical Product Outsourcing’s flagship issue, featuring the very first Top 30 Global Medical Device Companies report (this year’s version to be featured next month—stay tuned).
That year’s list of the elite was championed by global medical device, drug, and consumer products company Johnson & Johnson, which would remain at the top spot for over a decade. J&J’s $11.6 billion in medical device revenue now seems paltry compared to 2017’s leader—Medtronic plc—which touted $28.8 billion in sales.
Partially responsible for J&J’s windfall in 2003 resulted from Cordis Corporation’s (then a J&J business, now owned by Cardinal Health) April launch of a landmark treatment for coronary artery disease, and the first commercial device of its type—the Cypher drug-eluting stent, or DES for short.
Cordis’ Cypher DES was hailed as the first combination drug-device intended to help reduce restenosis (blockage) of a coronary artery treated with a stent. Restenosis was then—and continues to be—an enormous challenge in long-term stent implantations used for interventional cardiology treatment. To prevent it, Cypher was coated in sirolimus, a macrolide compound that is also used to prevent organ transplant rejection and treat LAM—a rare, progressive, and systemic lung disease.
“Cordis is very pleased to bring this remarkable and innovative treatment to patients, hospitals, and interventional cardiologists,” said then Johnson & Johnson Company Group Chairman Robert Croce upon Cypher’s U.S. launch. “Clinical evidence and experience with more than 50,000 patients treated to date in nearly 60 countries suggests the Cypher Stent represents the beginning of a new era in interventional cardiology—an era in which the combination of drugs and devices substantially improves patient outcomes.”
DES technology was regarded to be such a significant improvement on current bare metal stent technology that the Centers for Medicare and Medicaid services (CMS) approved Cypher’s incremental reimbursement in August 2002, a full nine months before J&J launched Cypher in the U.S. The new policy provided significant incremental reimbursement over and above 2003’s bare metal stent reimbursement levels—undoubtedly due to Cypher’s demonstrated sustained reduction in blockage incidence by over 90 percent in Cordis’ landmark U.S. SIRIUS trial.
A number of other medical technology giants were racing to commercialize their own DES products that year as well—J&J was simply the first to reach the U.S. market, granting it a sizeable head start. Around the same time J&J was gearing up to launch Cypher, Boston Scientific Corp. had achieved CE mark approval for its Taxus paclitaxel-eluting coronary stent. Taxus launched in the U.S. in March 2004, almost a full year behind Cypher. Paclitaxel, Boston Scientific’s drug of choice for the Taxus DES, is also used as a chemotherapy medication to treat a number of types of cancer.
“The approval of the Taxus system marks an important opportunity for clinicians in the United States,” said Gregg Stone, M.D., then vice chairman of the Cardiovascular Research Foundation at the Lenox Hill Heart and Vascular Institute in New York, and principal investigator of the Taxus IV clinical trial. “In paclitaxel, we now have a multi-functional drug that is safe and highly effective. The Taxus system has shown impressive results across a wide range of patients. Its performance has been particularly impressive in challenging cases such as patients with diabetes, small vessels, and long lesions.”
Guidant Corporation—which Boston Scientific acquired in 2015, after settling a lawsuit with J&J over the deal—had its own stent in the ring around that time, this one coated in everolimus, a sirolimus derivative used to prevent organ transplant rejection and treat renal cell cancer. However, rather than bringing its product to market itself, in February 2004 Guidant began a deal with Cordis to co-promote Cypher and assist the company in developing a Cypher stent utilizing Guidant’s stent delivery system. The agreement granted Guidant immediate entry into the U.S. DES market and settled ongoing stent-related patent disputes between the companies.
Medtronic was also in the midst of clinical trials for its own Endeavor phosphorylcholine-coated DES in 2003, but did not achieve FDA approval and launch of the device until 2008, though it was the first DES approved in four years. Phosphorylcholine, unlike the other drug compounds used previously, is exclusively meant to prevent coronary artery blockage.
Today, a majority of stents used in interventional cardiology procedures are coated in some antiproliferative pharmaceutical agent to prevent restenosis. J&J exited the DES market in 2011 due to safety concerns and fierce competition from rival products, but the market remains strong—according to Grand View Research, it was valued at $5.6 billion in 2015 with major players Boston Scientific, Medtronic, Abbott Laboratories, Biosensors International, Biotronik, Lepu Medical Technology, Terumo Medical, Cook Medical, Shandong JW Medical Systems, and Stentys leading the charge.