Maria Shepherd, President and Founder, Medi-Vantage01.30.18
MassMEDIC is the largest regional medical device association in the United States, with member companies ranging from the largest to the smallest medical technology innovators. The MassMEDIC Medtech Showcase, held in October every year, is a meeting devoted to the medical device industry.
Why This Is Important
Although the situation appears to be improving, there is still a lack of funding for medical device companies. This affects our personal health (we are all patients) and the health of our industry. Where else are the large, strategic companies going to find innovation and technology? While there are many reasons investment in our industry is difficult to obtain, organizations like MassMEDIC are stepping up to the plate to illustrate the ways in which innovation abounds in our business.
At the 2017 event, the industry trend toward digital health (Table 1) was well represented by two companies, although several more had digital features in their devices. Common Sensing makes smart devices focused on the drug delivery market. The company’s first product was developed with an emphasis on diabetes management. The second digital health firm, Augmentx, is an augmented reality digital therapy company currently focused on neuro-rehabilitation for stroke patients.

Table 1
Representing the cardiovascular device space were Bitome—a developer of a portable hydration monitor for managing congestive heart failure—and Protembis—a manufacturer of a cerebral protection system that deflects embolic material away from the brain during transcatheter aortic valve replacement (TAVR). Interventional cardiology is a medical specialty demanding innovation since cardiovascular disease is still so prevalent. It has been decades since Andreas Gruentzig, M.D., started this treatment sector by performing a heart catheterization on himself. (Who says interventional cardiologists aren’t risk takers?) Despite his extraordinary achievements, cardiovascular disease (including valve disease) is still the number one cause of death in the United States and minimally invasive cardiac valve procedures have come a long way. What started as the U.S. TVT registry in 2012 became three separate registries: TAVR, TMC (transcatheter mitral leaflet clip), and TMViV/TMViR (transcatheter mitral valve-in-valve therapy/transcatheter mitral valve-in-ring therapy)2 (Table 2).
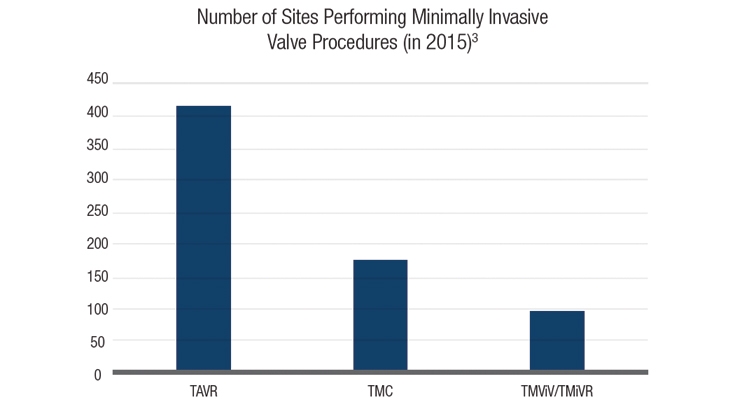
Table 2
Most interesting were the presentations from Summit Medical and Prapela, for uniquely different reasons. Summit Medical is intently focused on the EpiPen market. If there was ever a healthcare sector in desperate need of disruption, the epinephrine auto-injector market is it. At one point, Mylan (everyone recall the price gouging headlines?), the original manufacturer of the EpiPen—a drug delivery device used to address allergic reactions—controlled 95 percent of the epinephrine auto-injector market. When the company raised the price by 400 percent,4 it outraged both members of Congress and parents across the United States. Lesson learned. Since then, Mylan’s market share has dwindled to just over 71 percent as irate doctors seek alternate solutions for their patients.
On the other hand, Prapela is a completely new concept and was the winner of the MassMEDIC Best Overall Presentation Award. Talk about addressing an unmet need; technically, this device is a random (stochastic) vibration box helping to coax babies into calming themselves. For sleep-deprived parents, however, it is a device that gently stimulates their babies, improving relaxation and breathing rhythm during sleep.
A medical device helping babies to sleep better? This technology taps into an enormous market opportunity. Approximately 20 to 25 percent of babies match the definition for colic.5 Further, preterm births occur in about 12 percent of all pregnancies in the United States6—more than 470,000 babies a year. (Table 3)
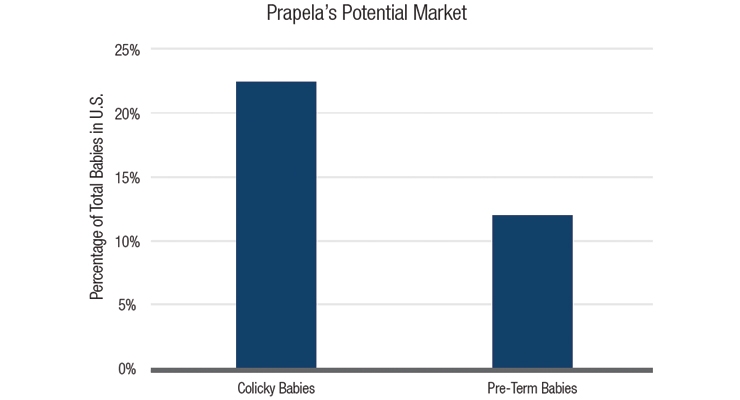
Table 3
The Medi-Vantage Perspective
We love entrepreneurs in medtech and want to see more of them growing in the Massachusetts ecosystem for startups. We applaud organizations like MassMEDIC, M2D2, and MassChallenge—all part of the start-up growth engine that continues to propel the Massachusetts base of medtech, diagnostic, and digital health entrepreneurs forward.
Get Involved in Innovation
The global decline in medtech entrepreneurship continues (see the November/December MPO Datawatch column “Market Pressures Have Squeezed Medtech Innovation”). Let’s reverse the curse. Entrepreneurs like those that presented at MassMEDIC need our support and we can help by attending these meetings to show support of the product pipeline that feeds us all.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy as well as innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.msbiv.com). She can be reached at 855-343-3100, ext. 102, or at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com
Why This Is Important
Although the situation appears to be improving, there is still a lack of funding for medical device companies. This affects our personal health (we are all patients) and the health of our industry. Where else are the large, strategic companies going to find innovation and technology? While there are many reasons investment in our industry is difficult to obtain, organizations like MassMEDIC are stepping up to the plate to illustrate the ways in which innovation abounds in our business.
At the 2017 event, the industry trend toward digital health (Table 1) was well represented by two companies, although several more had digital features in their devices. Common Sensing makes smart devices focused on the drug delivery market. The company’s first product was developed with an emphasis on diabetes management. The second digital health firm, Augmentx, is an augmented reality digital therapy company currently focused on neuro-rehabilitation for stroke patients.

Table 1
Representing the cardiovascular device space were Bitome—a developer of a portable hydration monitor for managing congestive heart failure—and Protembis—a manufacturer of a cerebral protection system that deflects embolic material away from the brain during transcatheter aortic valve replacement (TAVR). Interventional cardiology is a medical specialty demanding innovation since cardiovascular disease is still so prevalent. It has been decades since Andreas Gruentzig, M.D., started this treatment sector by performing a heart catheterization on himself. (Who says interventional cardiologists aren’t risk takers?) Despite his extraordinary achievements, cardiovascular disease (including valve disease) is still the number one cause of death in the United States and minimally invasive cardiac valve procedures have come a long way. What started as the U.S. TVT registry in 2012 became three separate registries: TAVR, TMC (transcatheter mitral leaflet clip), and TMViV/TMViR (transcatheter mitral valve-in-valve therapy/transcatheter mitral valve-in-ring therapy)2 (Table 2).

Table 2
Most interesting were the presentations from Summit Medical and Prapela, for uniquely different reasons. Summit Medical is intently focused on the EpiPen market. If there was ever a healthcare sector in desperate need of disruption, the epinephrine auto-injector market is it. At one point, Mylan (everyone recall the price gouging headlines?), the original manufacturer of the EpiPen—a drug delivery device used to address allergic reactions—controlled 95 percent of the epinephrine auto-injector market. When the company raised the price by 400 percent,4 it outraged both members of Congress and parents across the United States. Lesson learned. Since then, Mylan’s market share has dwindled to just over 71 percent as irate doctors seek alternate solutions for their patients.
On the other hand, Prapela is a completely new concept and was the winner of the MassMEDIC Best Overall Presentation Award. Talk about addressing an unmet need; technically, this device is a random (stochastic) vibration box helping to coax babies into calming themselves. For sleep-deprived parents, however, it is a device that gently stimulates their babies, improving relaxation and breathing rhythm during sleep.
A medical device helping babies to sleep better? This technology taps into an enormous market opportunity. Approximately 20 to 25 percent of babies match the definition for colic.5 Further, preterm births occur in about 12 percent of all pregnancies in the United States6—more than 470,000 babies a year. (Table 3)

Table 3
The Medi-Vantage Perspective
We love entrepreneurs in medtech and want to see more of them growing in the Massachusetts ecosystem for startups. We applaud organizations like MassMEDIC, M2D2, and MassChallenge—all part of the start-up growth engine that continues to propel the Massachusetts base of medtech, diagnostic, and digital health entrepreneurs forward.
Get Involved in Innovation
The global decline in medtech entrepreneurship continues (see the November/December MPO Datawatch column “Market Pressures Have Squeezed Medtech Innovation”). Let’s reverse the curse. Entrepreneurs like those that presented at MassMEDIC need our support and we can help by attending these meetings to show support of the product pipeline that feeds us all.
References
- http://bit.ly/mpo180101
- http://bit.ly/mpo180102
- http://bit.ly/mpo180103
- http://bit.ly/mpo180104
- http://bit.ly/mpo180105
- http://bit.ly/mpo180106
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy as well as innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.msbiv.com). She can be reached at 855-343-3100, ext. 102, or at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com



























