Mark Crawford, Contributing Writer10.05.17
Medical device manufacturers (MDMs) continue to make smaller, increasingly complex devices with unique geometries that require high-precision components—including extruded parts. For example, since minimally invasive surgical applications are highly preferred in the operating room, MDMs are pushing for tight-tolerance micro extrusion tubing to meet the performance specifications for these highly engineered devices. Smaller tubing with added functionality is also in demand, such as thinner wall thicknesses with improved hoop/burst strength, smaller inner diameters that can handle more lumens, and tubes with higher flexibility and “softness,” but also greater torque resistance.
Although advances have been made for smaller footprints, measurement systems, cutters, screw design, materials, and material feed (all of which improve the extrusion process and expand the limits of what it can do), it doesn’t take long to fall behind the technology curve. Extruders must constantly strive to improve their processes and push their boundaries to satisfy the ever-challenging needs of their MDM clients. This also requires deeper customer intimacy and in-depth knowledge of their internal processes. And, with the U.S. Food and Drug Administration (FDA) pushing regulatory responsibilities further down the supply chain, extruders must also be experts on their quality systems and FDA requirements.
It is no surprise, then, that MDMs are increasingly dependent on their extrusion providers to meet their needs for complexity, tolerance, quality, and compliance. In other words, they seek extruders who can provide complete (or nearly complete) device solutions, rather than just tubing. This reduces MDM risk, improves quality, and speeds up time to market.
“Customers are looking for strategic development partners, rather than just component suppliers,” said Ray Ledinsky, global market manager for Teleflex Medical OEM, a Gurnee, Ill.-based provider of custom-engineered extrusions for the medical device industry. “Finding strategic partners helps them shorten their supply chains, from compounding all the way through to the finished device.”
What OEMs Want
MDMs are fixated on speed—their first request to their extrusion providers is almost always quick lead time. But they also want high-quality tubing, especially with thinner walls and tighter tolerances on inner diameter/outer diameter (ID/OD), wall thickness, and length. Other common customer requests are softer durometer materials, higher strengths, and customized features such as specific degradation rates. Large-bore concentric extrusions for trans-catheter mitral valve repair are also in increased demand.
“Smaller sizes and tighter tolerances are trends we have been seeing over the last few years, which continue to challenge extrusion technology,” said Adam Nadeau, process technology R&D manager for Saint-Gobain Performance Plastics, a Portage, Wis.-based provider of molding and extrusion services for the medical device industry.
Ledinsky agreed.
“For example,” he said, “customers are asking for very precise quality requirements for cutting extremely stiff materials like polycarbonate or polyetheretherketone [PEEK].”
Extruders also report that customers are more focused on delivering top quality extruded products for applications where success depends on highly consistent tubing performance. “In devices like drug delivery pumps, for example, variations in dimensions, or even physical properties, can potentially keep the pump from working properly, which can impact the patient that depends on an accurate and steady drug dosage,” said Nadeau. “It is important to understand exactly what the need is first, and then create specifications that truly define the expectations for the tubing.”
OEMs also want better quality and validation, especially as products become more complex and challenging to make and the FDA continues to scrutinize supply chain integrity. With more guidance coming from the FDA and the EU, many extrusion suppliers are taking a harder look at how their products might be considered to be medical devices. With these increased regulatory complexities, and other risk considerations, MDMs are outsourcing more extrusion and assembly work to their supply chains, especially those vendors with top-notch quality systems in place.
“Customers are looking at global footprints to validate and produce their products at locations around the world,” said Bob Donohue, general manager for Natvar, a Wayne, Pa.-based provider of precision medical tubing solutions. “That, in conjunction with the raw material supply chain, is a common theme. OEMs want the material supply chain to be solid, with little to no risk of it being discontinued. This is a key factor in material selection.”
Another way to strengthen the supply chain is to shorten it. MDMs are always on the lookout for vertically integrated companies and vendors with a range of skills and solutions that can speed up decision-making, shorten lead times, improve efficiencies, and control costs. Extruders that are skilled in multiple areas, customize products when needed, and can find ways to meet a customer’s unique specifications are in high demand by MDMs. It’s even better when the solutions are relatively simple and don’t require major investments in technology—for example, preformed tubing.
“Thermoformed tubing is increasing in demand,” said Marcia Coulson, president of Eldon James Corporation, a Denver, Colo.-based manufacturer of standard and custom fittings, PVC-free tubing, and assemblies. “Consumers want the convenience of tubes that are pre-formed to route around components within their devices. As customers reduce the physical footprint of devices and machines, the tubes can be pre-formed to fit in smaller spaces. OEMs are also outsourcing more of the common assemblies so they can concentrate on developing new innovative medical devices.”
This relatively simple solution speeds up assembly and time to market, without additional complexity or cost—the two things MDMs want the most.
Recent Trends
There continues to be a shift from injection-molded parts to profile extrusion, a method that can produce shaped products of various configurations, including tubing. A pin or mandrel is placed inside the die to form the hollow sections. An air source is needed to help the product maintain its shape and not collapse during the process.
In the past, injection molding was the preferred process for producing medical parts with tight tolerances—something that could not be achieved with extrusion. “Now, however, with newer die designs, downstream equipment, vacuum sizing techniques, and overall technology catching up with injection molding, extrusion is now an option,” stated Donohue. Rotator cuff surgical devices are a good example of a product that now relies more on extrusion, rather than injection molding.
Micro extrusion capabilities continue to expand for both established medical device extrusion providers and new companies entering the market. Device and hardware miniaturization to support less-invasive procedures requires smaller extruded components. “Increasing device design complexity also requires the availability of very small diameter extruded tubing with multiple channels,” noted Chris Mazelin, western director of OEM sales and business development for Bentec Medical, a Woodland, Calif.-based designer and manufacturer of silicone-based medical devices and related component-level parts.
A current trend in the medical extrusion market is larger-diameter, thin-wall tubes for tight tolerance applications in cardiovascular and neurovascular applications. Most companies extrude thin-walled materials over a core. However, Apollo Medical Extrusion Technologies, a division of Spectrum Plastics Group, uses a free extrusion process (without a core) to make sub-ultra-thin-wall tubes.
“Free extrusion has several advantages,” said Joe Stephens, vice president of sales and marketing for Apollo Medical, a Sandy, Utah-based custom manufacturer of catheter tubing. “First, the customer does not have to incur the cost of the core material. Second, if using a core, getting the extrusion to adhere to the core requires the extruded material to run at a cooler temperature. This changes the physical characteristics of the raw material, which may have a negative impact on the performance of the product.”
New Technologies and Approaches
Advancements in manufacturing techniques have led to the incorporation of bioactive materials into extruded materials, both as a way to deliver drugs and to promote healing. For example, pharmaceutical ingredients can be combined with silicone products to provide a variety of benefits. Silicone has very consistent absorption and elution characteristics. Ultraviolet light curable silicones enable extruders to mix drugs into the silicone and extrude drug-embedded tubing without impacting the effectiveness of the drug. These are typically antibiotics or antimicrobials that can be released at controlled rates. This, however, requires careful monitoring of the drug filler mixing into the silicone to be certain it does not negatively impact the complete curing of the silicone and subsequent performance of the extruded tubing.
As extrusion moves from simple ID/OD tubing to more complex designs, such as 20-lumen tubes, simulation modeling has become standard for developing tooling that does not require much adjustment before it can produce production parts. This allows extrusion manufacturers to help customers be more competitive by reducing their time to market with a higher-quality product.
“Particularly with the multi-lumen constructions, geometries can vary from customer to customer, so simulation and understanding tooling design is very important for meeting these customized requests in a profitable way,” said Sonia Schwantes, extrusion specialist for Saint-Gobain Performance Plastics.
Also, as heart surgeries and other procedures shift toward catheterization-type procedures, the demand for highly specialized and complex tubing profiles has grown tremendously. Therefore, profile extruders must be current on the latest technologies (such as modeling software), as well as downstream take-off equipment such as cutters and pullers, which have improved the efficiency and speed of extrusion technologies.
“Through modeling of tooling, manufactures can ensure easier implementation of new tooling, with less scrap and tighter tolerances than previously thought,” added Charles Golub, R&D senior research engineer for Saint-Gobain Performance Plastics.
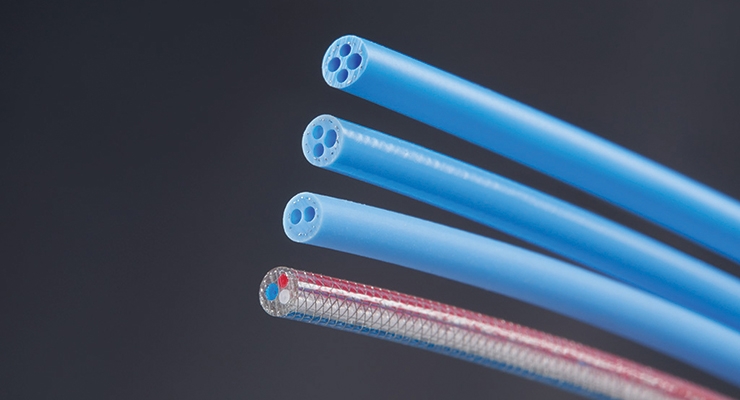
Micro extrusion methods can now make tight-tolerance tubing for a variety of demanding neurovascular interventional therapies and surgical applications. Micro-extruded tubing is ideal for IV cannulas, pediatric devices, infant micro catheters, guidewires, catheter leads, and any other devices where precision diameters and tight tolerances are necessary to achieve product objectives. Today micro extruders can produce multilayer tubing from up to four different polymer materials, with inner tubing diameters as small as 100 µm and wall thicknesses as thin as 50 µm.
“The latest micro-extrusion tubing and profiles can now help OEMs develop complex devices that provide therapies and treatments that were not previously possible,” said Donohue.
Over the last few years there has been a push toward taking a more data-driven approach to extrusion. For many companies, extrusion has been more of an art built on tribal knowledge, but that approach doesn’t work with the FDA’s increased scrutiny of documentation. A data-based program allows an extruder to create a more robust process that is not affected by material variation. This also meets the expectations of MDMs that their extruders are compliant with the necessary quality standards.
For extrusion, accurate gauging of tubing specs is critical for delivering the accuracy and tolerances that MDMs require. The top three critical specifications for medical tubing are OD, ID, and wall thickness. Accurate, in-process measurements of the tubing are required to provide wall balance and uniformity data in order to control the extrusion process.
Laser and ultrasonic technologies can be used to monitor these key parameters during extrusion. These data are then used by extruder management software to adjust extrusion machinery in real time, creating an automatic self-adjusting closed loop system. “This increases the ability of the extruder to extrude with greater accuracy and maintain tighter tolerances, even given today’s requirement for smaller and smaller tubing sizes,” noted Mazelin.
Material Impacts
Material improvements in plastics such as PEEK, polyurethanes, and polyolefins make them more attractive substitutes for long-established materials such as stainless steel. For example, new variations of PEEK are replacing stainless steel and other metals because PEEK’s strength and low coefficient of friction make it ideal for load-bearing applications such as implants.
“Materials such as PEEK and Veradel [apolyethersulfone] are changing the way medical device engineers are able to design products,” said Stephens. “These materials in multi-lumen configurations can replace and add additional capabilities to materials such as stainless steel and aluminum, in some applications. They are also magnetic resonance imaging (MRI) compatible, unlike metals.”
A new extrusion material that Apollo is working on is liquid crystal polymer (LCP), which has been marketed as a replacement for stainless steel. A key advantage of LCP over stainless steel is that it is nonferrous and also MRI-compatible.
“Although we have successfully extruded carbon fiber filled LCP, the natural grades are more challenging,” Stephens explained. “LCP is a highly crystalline material with a very high melt-flow index, which tends to make it more difficult to process.”
For any new material, the key to widespread acceptance is that it must act and perform like materials currently used in the medical device market. A top concern by MDMs is how a new material will impact the automation process. Over the years MDMs have invested significantly in automation for device assembly (complex and/or simple automation). They are willing to consider developing devices with advanced materials, but those materials must perform equally well in an automation process. “For example, there is a significant cost in developing and utilizing a new thermoplastic elastomer or thermoplastic polyurethane for a new or existing device,” said Donohue. “However, the cost goes up significantly if it cannot be used as efficiently with current automation. An example would be soft polyurethane materials, which typically do not work well in automation.”
An ongoing trend in the medical industry is MDMs moving away from polyvinyl chloride (PVC) and di-2-ethylhexyl phthalate (DEHP) tubing products and replacing them with more eco- and health-friendly tubing. To compete in this changing market, Eldon James has developed a tube that has the clarity and flexibility of PVC, but without the use of PVC, phthalates, or other additives. The company blended certain polyolefin plastics that are compatible to create a new resin for this specific need.
“PVC has always had a very low price point,” said Coulson. “In order to introduce our product and be competitive, we had to have a cost-effective solution that our customers would accept. Our new resin costs a little more, but the product meets the needs of the changing market away from PVC.”
Speed of Innovation
As medications and medical treatments advance, medical devices and delivery systems must keep pace. For example, high-moisture and light-sensitive tubing and profiles help protect unstable drug delivery systems. New developments in cancer drugs, and insulin delivery for diabetic patients, will require enhanced systems for getting these medications into the body, in ways that protect both the drug and the patient.
Sometimes the key to an extrusion process improvement is the material, rather than the machine.
“New materials may push production limits by allowing more torque strength or burst pressure with thinner walls and less material,” said Schwantes.
Extruders keep challenging their systems and know-how to push the limits, do things differently, and find the perfect formulation. For example, Eldon James challenged itself to produce PVC-free corrugated tubing. Many devices use corrugated tubing and MDMs want it to be PVC-free. “We found that traditional extrusion equipment required proprietary changes and, when combined with finding the right material, we were able to exceed the functional testing and meet the requirements for this replacement,” said Coulson.
Not all MDMs are comfortable or proficient with data-based decision-making. However, when they do make the transition, they are often startled by the clarity it brings to their operations. For example, Teleflex Medical OEM operates a customer solution center that utilizes its proprietary database of performance characteristic data. “Product decisions are based on data generated from testing, not opinion,” said Ledinsky. “We can interject our design for manufacturability expertise, providing a range of best practices, tolerances, and guidelines to ensure the user need is met, all while optimizing manufacturability. This provides an opportunity for deeper, more collaborative customer relationships.”
The Internet of Things/Industry 4.0 emphasizes machine-to-machine interconnection and communication for gathering performance data, using sensor technologies. This data is then analyzed, with subsequent adjustments made. When combined with design for manufacturability, these data-driven approaches optimize the production process and maximize quality and throughput.
For example, Saint-Gobain has developed a process called Compass Technology for silicone extrusion, which provides optimized formulations specific to the design application, precision tolerances for critical dimensions, and fluid system modeling for product design. The company recently had a customer that was beginning the submission process for a drug delivery pump when they began getting errors in the pump. A deep evaluation showed that the pump was so sensitive it was detecting minor changes in the modulus that were inherent in the tubing, due to raw material variation.
“We were able to develop a design of experiments to determine what the modulus range needed to be,” said Nadeau. “That allowed us to tighten that range down by using a custom compound to build a specification that would allow the pump to work accurately, without any costly redesign.”
Finally, sometimes it is not the latest technologies, machine learning, and data analytics that make the difference, but old-fashioned human-to-human communication.
A good example is Apollo’s “Engineering Days,” where medical device engineers work directly on the floor with Apollo’s extrusion engineers to short-cut and fine-tune the development process. “We collaborate with customers to make their tubing a repeatable and manufacturable process,” said Stephens. “Most companies have shaved weeks and months off the extrusion development using engineering days.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.
Although advances have been made for smaller footprints, measurement systems, cutters, screw design, materials, and material feed (all of which improve the extrusion process and expand the limits of what it can do), it doesn’t take long to fall behind the technology curve. Extruders must constantly strive to improve their processes and push their boundaries to satisfy the ever-challenging needs of their MDM clients. This also requires deeper customer intimacy and in-depth knowledge of their internal processes. And, with the U.S. Food and Drug Administration (FDA) pushing regulatory responsibilities further down the supply chain, extruders must also be experts on their quality systems and FDA requirements.
It is no surprise, then, that MDMs are increasingly dependent on their extrusion providers to meet their needs for complexity, tolerance, quality, and compliance. In other words, they seek extruders who can provide complete (or nearly complete) device solutions, rather than just tubing. This reduces MDM risk, improves quality, and speeds up time to market.
“Customers are looking for strategic development partners, rather than just component suppliers,” said Ray Ledinsky, global market manager for Teleflex Medical OEM, a Gurnee, Ill.-based provider of custom-engineered extrusions for the medical device industry. “Finding strategic partners helps them shorten their supply chains, from compounding all the way through to the finished device.”
What OEMs Want
MDMs are fixated on speed—their first request to their extrusion providers is almost always quick lead time. But they also want high-quality tubing, especially with thinner walls and tighter tolerances on inner diameter/outer diameter (ID/OD), wall thickness, and length. Other common customer requests are softer durometer materials, higher strengths, and customized features such as specific degradation rates. Large-bore concentric extrusions for trans-catheter mitral valve repair are also in increased demand.
“Smaller sizes and tighter tolerances are trends we have been seeing over the last few years, which continue to challenge extrusion technology,” said Adam Nadeau, process technology R&D manager for Saint-Gobain Performance Plastics, a Portage, Wis.-based provider of molding and extrusion services for the medical device industry.
Ledinsky agreed.
“For example,” he said, “customers are asking for very precise quality requirements for cutting extremely stiff materials like polycarbonate or polyetheretherketone [PEEK].”
Extruders also report that customers are more focused on delivering top quality extruded products for applications where success depends on highly consistent tubing performance. “In devices like drug delivery pumps, for example, variations in dimensions, or even physical properties, can potentially keep the pump from working properly, which can impact the patient that depends on an accurate and steady drug dosage,” said Nadeau. “It is important to understand exactly what the need is first, and then create specifications that truly define the expectations for the tubing.”
OEMs also want better quality and validation, especially as products become more complex and challenging to make and the FDA continues to scrutinize supply chain integrity. With more guidance coming from the FDA and the EU, many extrusion suppliers are taking a harder look at how their products might be considered to be medical devices. With these increased regulatory complexities, and other risk considerations, MDMs are outsourcing more extrusion and assembly work to their supply chains, especially those vendors with top-notch quality systems in place.
“Customers are looking at global footprints to validate and produce their products at locations around the world,” said Bob Donohue, general manager for Natvar, a Wayne, Pa.-based provider of precision medical tubing solutions. “That, in conjunction with the raw material supply chain, is a common theme. OEMs want the material supply chain to be solid, with little to no risk of it being discontinued. This is a key factor in material selection.”
Another way to strengthen the supply chain is to shorten it. MDMs are always on the lookout for vertically integrated companies and vendors with a range of skills and solutions that can speed up decision-making, shorten lead times, improve efficiencies, and control costs. Extruders that are skilled in multiple areas, customize products when needed, and can find ways to meet a customer’s unique specifications are in high demand by MDMs. It’s even better when the solutions are relatively simple and don’t require major investments in technology—for example, preformed tubing.
“Thermoformed tubing is increasing in demand,” said Marcia Coulson, president of Eldon James Corporation, a Denver, Colo.-based manufacturer of standard and custom fittings, PVC-free tubing, and assemblies. “Consumers want the convenience of tubes that are pre-formed to route around components within their devices. As customers reduce the physical footprint of devices and machines, the tubes can be pre-formed to fit in smaller spaces. OEMs are also outsourcing more of the common assemblies so they can concentrate on developing new innovative medical devices.”
This relatively simple solution speeds up assembly and time to market, without additional complexity or cost—the two things MDMs want the most.
Recent Trends
There continues to be a shift from injection-molded parts to profile extrusion, a method that can produce shaped products of various configurations, including tubing. A pin or mandrel is placed inside the die to form the hollow sections. An air source is needed to help the product maintain its shape and not collapse during the process.
In the past, injection molding was the preferred process for producing medical parts with tight tolerances—something that could not be achieved with extrusion. “Now, however, with newer die designs, downstream equipment, vacuum sizing techniques, and overall technology catching up with injection molding, extrusion is now an option,” stated Donohue. Rotator cuff surgical devices are a good example of a product that now relies more on extrusion, rather than injection molding.
Micro extrusion capabilities continue to expand for both established medical device extrusion providers and new companies entering the market. Device and hardware miniaturization to support less-invasive procedures requires smaller extruded components. “Increasing device design complexity also requires the availability of very small diameter extruded tubing with multiple channels,” noted Chris Mazelin, western director of OEM sales and business development for Bentec Medical, a Woodland, Calif.-based designer and manufacturer of silicone-based medical devices and related component-level parts.
A current trend in the medical extrusion market is larger-diameter, thin-wall tubes for tight tolerance applications in cardiovascular and neurovascular applications. Most companies extrude thin-walled materials over a core. However, Apollo Medical Extrusion Technologies, a division of Spectrum Plastics Group, uses a free extrusion process (without a core) to make sub-ultra-thin-wall tubes.
“Free extrusion has several advantages,” said Joe Stephens, vice president of sales and marketing for Apollo Medical, a Sandy, Utah-based custom manufacturer of catheter tubing. “First, the customer does not have to incur the cost of the core material. Second, if using a core, getting the extrusion to adhere to the core requires the extruded material to run at a cooler temperature. This changes the physical characteristics of the raw material, which may have a negative impact on the performance of the product.”
New Technologies and Approaches
Advancements in manufacturing techniques have led to the incorporation of bioactive materials into extruded materials, both as a way to deliver drugs and to promote healing. For example, pharmaceutical ingredients can be combined with silicone products to provide a variety of benefits. Silicone has very consistent absorption and elution characteristics. Ultraviolet light curable silicones enable extruders to mix drugs into the silicone and extrude drug-embedded tubing without impacting the effectiveness of the drug. These are typically antibiotics or antimicrobials that can be released at controlled rates. This, however, requires careful monitoring of the drug filler mixing into the silicone to be certain it does not negatively impact the complete curing of the silicone and subsequent performance of the extruded tubing.
As extrusion moves from simple ID/OD tubing to more complex designs, such as 20-lumen tubes, simulation modeling has become standard for developing tooling that does not require much adjustment before it can produce production parts. This allows extrusion manufacturers to help customers be more competitive by reducing their time to market with a higher-quality product.
“Particularly with the multi-lumen constructions, geometries can vary from customer to customer, so simulation and understanding tooling design is very important for meeting these customized requests in a profitable way,” said Sonia Schwantes, extrusion specialist for Saint-Gobain Performance Plastics.
Also, as heart surgeries and other procedures shift toward catheterization-type procedures, the demand for highly specialized and complex tubing profiles has grown tremendously. Therefore, profile extruders must be current on the latest technologies (such as modeling software), as well as downstream take-off equipment such as cutters and pullers, which have improved the efficiency and speed of extrusion technologies.
“Through modeling of tooling, manufactures can ensure easier implementation of new tooling, with less scrap and tighter tolerances than previously thought,” added Charles Golub, R&D senior research engineer for Saint-Gobain Performance Plastics.
Micro extrusion methods can now make tight-tolerance tubing for a variety of demanding neurovascular interventional therapies and surgical applications. Micro-extruded tubing is ideal for IV cannulas, pediatric devices, infant micro catheters, guidewires, catheter leads, and any other devices where precision diameters and tight tolerances are necessary to achieve product objectives. Today micro extruders can produce multilayer tubing from up to four different polymer materials, with inner tubing diameters as small as 100 µm and wall thicknesses as thin as 50 µm.
“The latest micro-extrusion tubing and profiles can now help OEMs develop complex devices that provide therapies and treatments that were not previously possible,” said Donohue.
Over the last few years there has been a push toward taking a more data-driven approach to extrusion. For many companies, extrusion has been more of an art built on tribal knowledge, but that approach doesn’t work with the FDA’s increased scrutiny of documentation. A data-based program allows an extruder to create a more robust process that is not affected by material variation. This also meets the expectations of MDMs that their extruders are compliant with the necessary quality standards.
For extrusion, accurate gauging of tubing specs is critical for delivering the accuracy and tolerances that MDMs require. The top three critical specifications for medical tubing are OD, ID, and wall thickness. Accurate, in-process measurements of the tubing are required to provide wall balance and uniformity data in order to control the extrusion process.
Laser and ultrasonic technologies can be used to monitor these key parameters during extrusion. These data are then used by extruder management software to adjust extrusion machinery in real time, creating an automatic self-adjusting closed loop system. “This increases the ability of the extruder to extrude with greater accuracy and maintain tighter tolerances, even given today’s requirement for smaller and smaller tubing sizes,” noted Mazelin.
Material Impacts
Material improvements in plastics such as PEEK, polyurethanes, and polyolefins make them more attractive substitutes for long-established materials such as stainless steel. For example, new variations of PEEK are replacing stainless steel and other metals because PEEK’s strength and low coefficient of friction make it ideal for load-bearing applications such as implants.
“Materials such as PEEK and Veradel [apolyethersulfone] are changing the way medical device engineers are able to design products,” said Stephens. “These materials in multi-lumen configurations can replace and add additional capabilities to materials such as stainless steel and aluminum, in some applications. They are also magnetic resonance imaging (MRI) compatible, unlike metals.”
A new extrusion material that Apollo is working on is liquid crystal polymer (LCP), which has been marketed as a replacement for stainless steel. A key advantage of LCP over stainless steel is that it is nonferrous and also MRI-compatible.
“Although we have successfully extruded carbon fiber filled LCP, the natural grades are more challenging,” Stephens explained. “LCP is a highly crystalline material with a very high melt-flow index, which tends to make it more difficult to process.”
For any new material, the key to widespread acceptance is that it must act and perform like materials currently used in the medical device market. A top concern by MDMs is how a new material will impact the automation process. Over the years MDMs have invested significantly in automation for device assembly (complex and/or simple automation). They are willing to consider developing devices with advanced materials, but those materials must perform equally well in an automation process. “For example, there is a significant cost in developing and utilizing a new thermoplastic elastomer or thermoplastic polyurethane for a new or existing device,” said Donohue. “However, the cost goes up significantly if it cannot be used as efficiently with current automation. An example would be soft polyurethane materials, which typically do not work well in automation.”
An ongoing trend in the medical industry is MDMs moving away from polyvinyl chloride (PVC) and di-2-ethylhexyl phthalate (DEHP) tubing products and replacing them with more eco- and health-friendly tubing. To compete in this changing market, Eldon James has developed a tube that has the clarity and flexibility of PVC, but without the use of PVC, phthalates, or other additives. The company blended certain polyolefin plastics that are compatible to create a new resin for this specific need.
“PVC has always had a very low price point,” said Coulson. “In order to introduce our product and be competitive, we had to have a cost-effective solution that our customers would accept. Our new resin costs a little more, but the product meets the needs of the changing market away from PVC.”
Speed of Innovation
As medications and medical treatments advance, medical devices and delivery systems must keep pace. For example, high-moisture and light-sensitive tubing and profiles help protect unstable drug delivery systems. New developments in cancer drugs, and insulin delivery for diabetic patients, will require enhanced systems for getting these medications into the body, in ways that protect both the drug and the patient.
Sometimes the key to an extrusion process improvement is the material, rather than the machine.
“New materials may push production limits by allowing more torque strength or burst pressure with thinner walls and less material,” said Schwantes.
Extruders keep challenging their systems and know-how to push the limits, do things differently, and find the perfect formulation. For example, Eldon James challenged itself to produce PVC-free corrugated tubing. Many devices use corrugated tubing and MDMs want it to be PVC-free. “We found that traditional extrusion equipment required proprietary changes and, when combined with finding the right material, we were able to exceed the functional testing and meet the requirements for this replacement,” said Coulson.
Not all MDMs are comfortable or proficient with data-based decision-making. However, when they do make the transition, they are often startled by the clarity it brings to their operations. For example, Teleflex Medical OEM operates a customer solution center that utilizes its proprietary database of performance characteristic data. “Product decisions are based on data generated from testing, not opinion,” said Ledinsky. “We can interject our design for manufacturability expertise, providing a range of best practices, tolerances, and guidelines to ensure the user need is met, all while optimizing manufacturability. This provides an opportunity for deeper, more collaborative customer relationships.”
The Internet of Things/Industry 4.0 emphasizes machine-to-machine interconnection and communication for gathering performance data, using sensor technologies. This data is then analyzed, with subsequent adjustments made. When combined with design for manufacturability, these data-driven approaches optimize the production process and maximize quality and throughput.
For example, Saint-Gobain has developed a process called Compass Technology for silicone extrusion, which provides optimized formulations specific to the design application, precision tolerances for critical dimensions, and fluid system modeling for product design. The company recently had a customer that was beginning the submission process for a drug delivery pump when they began getting errors in the pump. A deep evaluation showed that the pump was so sensitive it was detecting minor changes in the modulus that were inherent in the tubing, due to raw material variation.
“We were able to develop a design of experiments to determine what the modulus range needed to be,” said Nadeau. “That allowed us to tighten that range down by using a custom compound to build a specification that would allow the pump to work accurately, without any costly redesign.”
Finally, sometimes it is not the latest technologies, machine learning, and data analytics that make the difference, but old-fashioned human-to-human communication.
A good example is Apollo’s “Engineering Days,” where medical device engineers work directly on the floor with Apollo’s extrusion engineers to short-cut and fine-tune the development process. “We collaborate with customers to make their tubing a repeatable and manufacturable process,” said Stephens. “Most companies have shaved weeks and months off the extrusion development using engineering days.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.