Maria Shepherd, Medi-Vantage09.01.17
Medical device product innovation and pricing strategy must be closely linked. In medtech product development, the three most important questions are:
Defining the unmet need is only the first step. The next step is ensuring that the medical device product development process meets the unmet need, fits within the clinical workflow, and overcomes every predictable obstacle to use. Sustainability is assessed through medical device market research that identifies the market positions of direct and indirect competitors. Most critical (and most difficult) is naming the payer for the product, how much it is worth to them, and why?
Why This Is Important
It is expensive to bring a medical device to market. A 2010 Stanford study1 identified the average cost to bring a 510(k) product from concept to market at $31 million. Greater than 77 percent of the cost—approximately $24 million—was consumed on regulatory and FDA-related activities. The cost of a PMA averaged $94 million, with $75 million spent on FDA-linked stages—nearly 80 percent of the total amount of bringing medtech devices to market launch (Table 1).
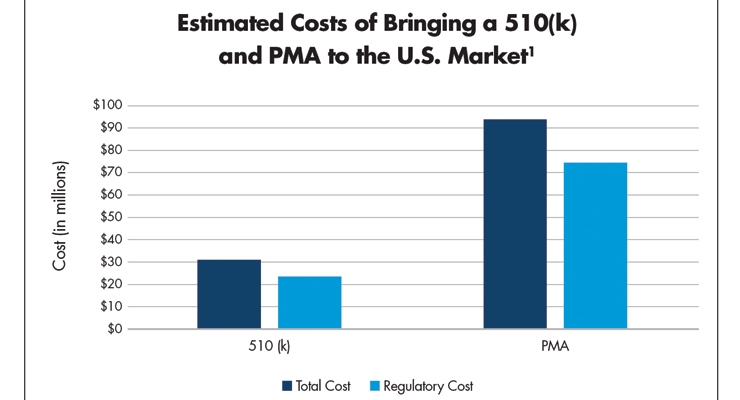
Table 1
Medical Device Pricing Strategy and Conjoint Analysis
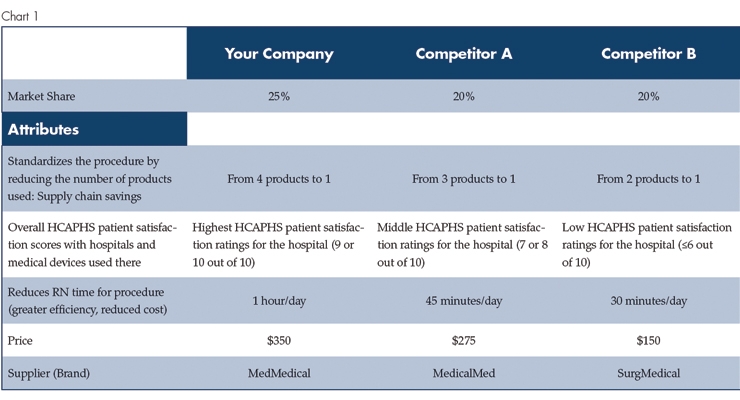
Want to know how your customers are thinking when it comes to making product or pricing decisions? Conjoint analysis (also called Discrete Choice Analysis or sometimes MaxDiff) is an advanced market research strategy used in medtech to understand how end users make decisions and what they really value in medical device products and services. In conjoint analysis, we present clinicians and hospital administrators with choices to identify the drivers for the choices they make. The best part? You and your team end up with a market simulator (Chart 1), giving you the answers to questions like:
What Will a Market Simulator Do for Your Medtech Product Development Team?
The market simulator is an important tool that is the output of medtech conjoint analysis. The simulator is used to convert statistically significant conjoint data into a tool medtech executive management wants and needs—the ability to simulate market choices. A market simulator lets the product development team conduct “What-if?” scenarios to investigate new product designs, product positioning, and price strategies. It helps the team identify market segments (i.e., groups of clinicians, hospital administrators, or patients with unique and targetable preferences).
HCAHPS Survey Experience Scores
If you haven’t heard about the HCAHPS survey, you will soon. HCAHPS is the first national, standardized, publicly reported survey of patients’ perspectives of hospital care. Hospitals are publicly reported on the Medicare website at www.medicare.gov/hospitalcompare. Two global measures capturing the overall rating of patient satisfaction with the hospital (and the medical devices they encounter there) are ranked on a 0 to 10 scale.
Conclusion and Recommendations
A conjoint survey can be expensive, but with the cost of bringing a medical device to market assessed at $30 million to $94 million, the cost of a conjoint and the confidence in decision making it offers is short money. In any new medical device, explore product design configurations and pricing strategies to reduce risk involved with bringing that medical device to market.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. Her career included a role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic. She was also director of marketing for Philips Medical and held senior management roles at Boston Scientific Inc. In 2007, she founded Medi-Vantage. Medi-Vantage provides marketing, business, and product development strategy research for the medical device, diagnostics, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught medtech marketing and product development courses and is a member of the Aligo Medtech Investment Committee. She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.
- Is there an unmet need that can be answered with a medical device and what is it?
- Is the unmet need sustainable and for how long?
- Who (e.g., payer, hospital administrator, surgeon owner of an ASC) is going to pay for this product, how much, and why?
Defining the unmet need is only the first step. The next step is ensuring that the medical device product development process meets the unmet need, fits within the clinical workflow, and overcomes every predictable obstacle to use. Sustainability is assessed through medical device market research that identifies the market positions of direct and indirect competitors. Most critical (and most difficult) is naming the payer for the product, how much it is worth to them, and why?
Why This Is Important
It is expensive to bring a medical device to market. A 2010 Stanford study1 identified the average cost to bring a 510(k) product from concept to market at $31 million. Greater than 77 percent of the cost—approximately $24 million—was consumed on regulatory and FDA-related activities. The cost of a PMA averaged $94 million, with $75 million spent on FDA-linked stages—nearly 80 percent of the total amount of bringing medtech devices to market launch (Table 1).

Table 1
Medical Device Pricing Strategy and Conjoint Analysis
Want to know how your customers are thinking when it comes to making product or pricing decisions? Conjoint analysis (also called Discrete Choice Analysis or sometimes MaxDiff) is an advanced market research strategy used in medtech to understand how end users make decisions and what they really value in medical device products and services. In conjoint analysis, we present clinicians and hospital administrators with choices to identify the drivers for the choices they make. The best part? You and your team end up with a market simulator (Chart 1), giving you the answers to questions like:
- Should we build in more features, or reduce the price?
- Which of these changes will take the most market share from our competitors?
- If we claim improved patient outcomes, what price premium will they pay?

What Will a Market Simulator Do for Your Medtech Product Development Team?
The market simulator is an important tool that is the output of medtech conjoint analysis. The simulator is used to convert statistically significant conjoint data into a tool medtech executive management wants and needs—the ability to simulate market choices. A market simulator lets the product development team conduct “What-if?” scenarios to investigate new product designs, product positioning, and price strategies. It helps the team identify market segments (i.e., groups of clinicians, hospital administrators, or patients with unique and targetable preferences).
HCAHPS Survey Experience Scores
If you haven’t heard about the HCAHPS survey, you will soon. HCAHPS is the first national, standardized, publicly reported survey of patients’ perspectives of hospital care. Hospitals are publicly reported on the Medicare website at www.medicare.gov/hospitalcompare. Two global measures capturing the overall rating of patient satisfaction with the hospital (and the medical devices they encounter there) are ranked on a 0 to 10 scale.
Conclusion and Recommendations
A conjoint survey can be expensive, but with the cost of bringing a medical device to market assessed at $30 million to $94 million, the cost of a conjoint and the confidence in decision making it offers is short money. In any new medical device, explore product design configurations and pricing strategies to reduce risk involved with bringing that medical device to market.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. Her career included a role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic. She was also director of marketing for Philips Medical and held senior management roles at Boston Scientific Inc. In 2007, she founded Medi-Vantage. Medi-Vantage provides marketing, business, and product development strategy research for the medical device, diagnostics, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught medtech marketing and product development courses and is a member of the Aligo Medtech Investment Committee. She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.




























