David Novotny, Novella Clinical09.01.17
By the time one reaches age 75, each of his or her four heart valves will have opened and closed approximately 3 billion times. But aging and illness cause some heart valves to stiffen or flop. Although mild valve damage may be treated with medication such as ACE inhibitors, beta-blockers, or vasodilators to reduce the risk of further damage or to manage symptoms, more significant damage may require repair or a replacement valve.
As a result of successful innovations in interventional cardiovascular devices, physicians now have many options with which they can repair or replace faulty heart valves.
The cardiologist and cardiac surgeon will choose either valve repair or replacement based on a number of factors, including the patient’s overall health, the valve itself, and type and severity of the damage.
Surgical Options
Surgery is usually selected to address congenital defects in valves, especially for mitral valves. Repairs include opening narrowed or tight valves caused by thickened leaflets; inserting a supportive device that surrounds a leaky valve; reshaping a valve’s leaflet; decalcifying leaflets; repairing the structural support of a valve; or repairing any tears or holes in leaflets.
When repair is not an option, the valve might be replaced with either a mechanical or bioprosthetic valve. Mechanical valves are made with plastic, carbon, or metal, and are quite sturdy, as they are designed to last for the rest of the patient’s life. Because platelets in the blood often stick to such valves, anticoagulation medication is prescribed to help prevent blood clots.
Bioprosthetic valves are made from animal tissue and are referred to as xenograft, whereas human tissue from a donated heart is known as an allograft or homograft. If the tissue comes from the patient, it is called an autograft. Tissue-based valves are not as durable as mechanical devices, lasting only about seven to 15 years based on current research and implantation data. But these valves, for the most part, also do not require ongoing anti-coagulants after surgery.
The Development of TAVR
One of the most common valve defects is aortic stenosis, or the narrowing of the aortic valve. Globally, about 12.4 percent of people aged 75 and older have aortic stenosis, including 3.4 percent
 with severe aortic stenosis.1 For decades, the standard of care for patients with severe aortic stenosis has been surgical aortic valve replacement. These procedures, however, are not an option for up to 40 percent of patients due to their high surgical risk.
with severe aortic stenosis.1 For decades, the standard of care for patients with severe aortic stenosis has been surgical aortic valve replacement. These procedures, however, are not an option for up to 40 percent of patients due to their high surgical risk.
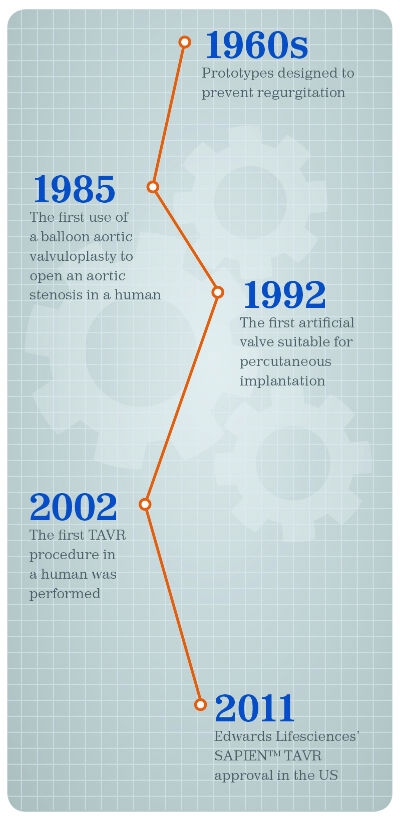
Less invasive methods to replace valves grew from prototype devices designed to prevent regurgitation in the mid-1960s, culminating with the first use of a balloon aortic valvuloplasty in a human with aortic stenosis in 1985. Researchers built the first artificial valve suitable for percutaneous implantation in the early 1990s and about a decade later, the first transcatheter aortic valve replacement (TAVR) procedure in a human was performed.
Today, an estimated 100,000 North American and 190,000 European patients annually undergo the procedure.2 Commercial sales for TAVR devices is anticipated to reach nearly $4 billion by 2020.3
A Dynamic Market Meets Evolving Challenges
The success of TAVR has created an opportunity for advancing transcatheter approaches for patients in need of mitral valve replacement. Several companies are developing transcatheter mitral valve repair (TMVR) devices, with the first receiving U.S. Food and Drug Administration approval in 2013. The pace of TMVR clinical trials has not yet matched that of TAVR due to the complex structure of mitral valves and the need for devices to serve a larger patient population with more diverse conditions and comorbidities.
Along with testing new TAVR and TMVR devices and procedures, sponsors are examining existing devices in new patient populations in an effort to improve surgical precision and safety, enhance patient outcomes, and boost device durability. Actually, durability and its implications for device use in lower-risk populations has been of keen interest in the last two years, during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Last year, one study suggested TAVR may not be suitable for younger patients and those at lower risk.4 Though it was a small study and examined only three first-generation devices, investigators found moderate to severe degeneration in 35 of 100 patients who survived at least five years after their procedures. The percentage of patients without deterioration decreased from about 80 at year six to less than 40 by year eight. Just two of 387 patients survived 10 years after the intervention.
Experts noted the results may not apply to newer devices, which are evolving in strength and design, but caution may be appropriate in considering TAVR for lower risk populations.5
Despite progress addressing new frontiers, old challenges remain to be conquered and offer new strategic opportunities for success to manufacturers and ultimately, the patient. The cardiovascular device space still needs to account for the variability in each patient’s valve structure, health, and complex positioning and anchoring needs. Trials will need to examine how any investigational device, particularly TMVR devices, prevents obstructions and perivalvular leaks while collecting data that shows valve repair or replacement prolongs patient survival and improves quality of life.
References
David Novotny is senior vice president of Novella Clinical’s Medical Device & Diagnostics Division, overseeing the Cardiovascular Therapies delivery unit. He has nearly 20 years of clinical research experience in the hospital, clinical research organization, medical device, and biopharmaceutical environments. Novotny is responsible for the oversight, strategy, and operational success of all medical device and diagnostics sponsor programs globally at Novella, including clinical execution and governance management.
As a result of successful innovations in interventional cardiovascular devices, physicians now have many options with which they can repair or replace faulty heart valves.
The cardiologist and cardiac surgeon will choose either valve repair or replacement based on a number of factors, including the patient’s overall health, the valve itself, and type and severity of the damage.
Surgical Options
Surgery is usually selected to address congenital defects in valves, especially for mitral valves. Repairs include opening narrowed or tight valves caused by thickened leaflets; inserting a supportive device that surrounds a leaky valve; reshaping a valve’s leaflet; decalcifying leaflets; repairing the structural support of a valve; or repairing any tears or holes in leaflets.
When repair is not an option, the valve might be replaced with either a mechanical or bioprosthetic valve. Mechanical valves are made with plastic, carbon, or metal, and are quite sturdy, as they are designed to last for the rest of the patient’s life. Because platelets in the blood often stick to such valves, anticoagulation medication is prescribed to help prevent blood clots.
Bioprosthetic valves are made from animal tissue and are referred to as xenograft, whereas human tissue from a donated heart is known as an allograft or homograft. If the tissue comes from the patient, it is called an autograft. Tissue-based valves are not as durable as mechanical devices, lasting only about seven to 15 years based on current research and implantation data. But these valves, for the most part, also do not require ongoing anti-coagulants after surgery.
The Development of TAVR
One of the most common valve defects is aortic stenosis, or the narrowing of the aortic valve. Globally, about 12.4 percent of people aged 75 and older have aortic stenosis, including 3.4 percent

Less invasive methods to replace valves grew from prototype devices designed to prevent regurgitation in the mid-1960s, culminating with the first use of a balloon aortic valvuloplasty in a human with aortic stenosis in 1985. Researchers built the first artificial valve suitable for percutaneous implantation in the early 1990s and about a decade later, the first transcatheter aortic valve replacement (TAVR) procedure in a human was performed.
Today, an estimated 100,000 North American and 190,000 European patients annually undergo the procedure.2 Commercial sales for TAVR devices is anticipated to reach nearly $4 billion by 2020.3
A Dynamic Market Meets Evolving Challenges
The success of TAVR has created an opportunity for advancing transcatheter approaches for patients in need of mitral valve replacement. Several companies are developing transcatheter mitral valve repair (TMVR) devices, with the first receiving U.S. Food and Drug Administration approval in 2013. The pace of TMVR clinical trials has not yet matched that of TAVR due to the complex structure of mitral valves and the need for devices to serve a larger patient population with more diverse conditions and comorbidities.
Along with testing new TAVR and TMVR devices and procedures, sponsors are examining existing devices in new patient populations in an effort to improve surgical precision and safety, enhance patient outcomes, and boost device durability. Actually, durability and its implications for device use in lower-risk populations has been of keen interest in the last two years, during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Last year, one study suggested TAVR may not be suitable for younger patients and those at lower risk.4 Though it was a small study and examined only three first-generation devices, investigators found moderate to severe degeneration in 35 of 100 patients who survived at least five years after their procedures. The percentage of patients without deterioration decreased from about 80 at year six to less than 40 by year eight. Just two of 387 patients survived 10 years after the intervention.
Experts noted the results may not apply to newer devices, which are evolving in strength and design, but caution may be appropriate in considering TAVR for lower risk populations.5
Despite progress addressing new frontiers, old challenges remain to be conquered and offer new strategic opportunities for success to manufacturers and ultimately, the patient. The cardiovascular device space still needs to account for the variability in each patient’s valve structure, health, and complex positioning and anchoring needs. Trials will need to examine how any investigational device, particularly TMVR devices, prevents obstructions and perivalvular leaks while collecting data that shows valve repair or replacement prolongs patient survival and improves quality of life.
References
- Osnabrugge, R., et al. Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2013;62(11):1002-1012.
- Bourantas, C. and Serruys P. Evolution of Transcatheter Aortic Valve Replacement. Circulation Research. 2014; 114: 1037-1051 doi: 10.1161/CIRCRESAHA.114.302292. bit.ly/mpo091701
- Technavio. Global Transcatheter Aortic Valve Replacement Market to Generate Close to USD 4 Billion by 2020, says Technavio. Press release. Feb. 3, 2016.
- Lou, N. “EuroPCR: Degenerated TAVR Not Uncommon by 10 Years.” MedPage Today. May 17, 2016. bit.ly/mpo091702
- Dvir, D. First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years after implantation. Presented at: EuroPCR 2016. May 17, 2016. Paris, France. bit.ly/mpo091703
David Novotny is senior vice president of Novella Clinical’s Medical Device & Diagnostics Division, overseeing the Cardiovascular Therapies delivery unit. He has nearly 20 years of clinical research experience in the hospital, clinical research organization, medical device, and biopharmaceutical environments. Novotny is responsible for the oversight, strategy, and operational success of all medical device and diagnostics sponsor programs globally at Novella, including clinical execution and governance management.