Matthew Therrien, Northeast Regional Manager, RJG Inc.05.01.17
With increasingly lean labor budgets and project volume not slowing down, organizations should take advantage of the expertise and experience their supply chain has to offer. Building stronger relationships through a team approach can yield improved knowledge sharing, increased awareness of project constraints/boundaries, and identification of common goals. Ultimately, all stakeholders benefit when projects are completed at or before the deadline.
With specific regard to plastic molded components in a medical device assembly, there are effective risk management approaches that the OEM and outsourcing suppliers can leverage to minimize the probability of recalls. Having an increased level of confidence in the molded components of an assembly provides value by enabling the OEM to focus on the overall performance of the medical device.
As it relates to “cost of quality”—the supply chain’s ability to deliver good parts into the product stream with minimal variation at a cost-effective price—a major task for procurement is to find the value match, not just shop for “piece part price.” With each new project, whether a next-generation device or an enhancement to a legacy product, what’s most important needs to be identified. Further, how best to accomplish the following tasks needs to be considered:
Technical collaboration in business requires the participating companies to become active learning organizations that employ a performance culture that promotes ongoing learning by putting the methods into practice and provides opportunities for growth. To get started, a CORE team must be established, background knowledge developed, and the practical execution of the methods learned.
Identifying the Core Team
The CORE team consists of a champion/coach from each company or department (internal and external stakeholders) who are responsible for transferring learned knowledge to support the common goal of finding a feasible solution to a problem or opportunity through data-driven decisions. It is a best practice approach to transfer knowledge from training and implementation through execution, making the team deep and wide.
The team is responsible for its performance and communication back to each respective department as resources are needed to satisfy the project’s established/approved process flow map.
Not every member of the CORE team is an expert, but the strategy drives the right behavior for the group. Every stakeholder through which the part/device passes during its development/production cycle must have a representative on the team at the beginning. This could include, but is not limited to, the end customer, engineering, quality, assembly and/or automation, molding, manufacturing, and even key members of the supply chain.
Collaboration begins when an honest assessment can be performed to identify strengths and weaknesses. From that analysis, organizations can learn and apply a systematic approach that can be customized for each project, developing a flexible process flow map and establishing an effective method for checks and balances.
Not all projects require full collaboration. By performing a data-driven risk assessment, one can accurately understand the desired deliverables of a project. Evaluation of the risk assessment results (based on a defined scale, considering multiple categories) determines how much collaboration is needed. Assigning a measurement allows organizations to know what’s required and facilitate proper allocation of resources.
Throughout a project, each resource team might have a varying percentage of participation required, freeing them up to perform other job responsibilities and functions.
Building the Knowledge Base
When the investment in individual training is applied and put into practice to educate the cross-functional CORE teams that are established for each project, the core competency learning/experience can be optimized across the entire organization. By no longer working in silos, these teams eliminate the “pass the baton” mentality. Certain elements of CORE teams can be taught and put into practice to enable this to become a reality. It doesn’t happen overnight, but team members will learn how to develop a roadmap, executable plan, and document results for each project.
In many cases, these silos (or geographic distances) are encountered between functional groups. “Throwing it over the wall” to the next department is not a strategy for success. Rather, cross functional education and training with these departments (internal and external) increases communication and data-driven decision making, improving the process flow, understanding the constraints, and documenting the concessions. The first step is an understanding of part/product development “from the plastics point of view,” accomplished through concurrent project execution and method training for future success.
For plastic components in a device assembly, understanding the fundamental principles of injection molding helps the entire CORE team understand the elements and processes required to produce the part.
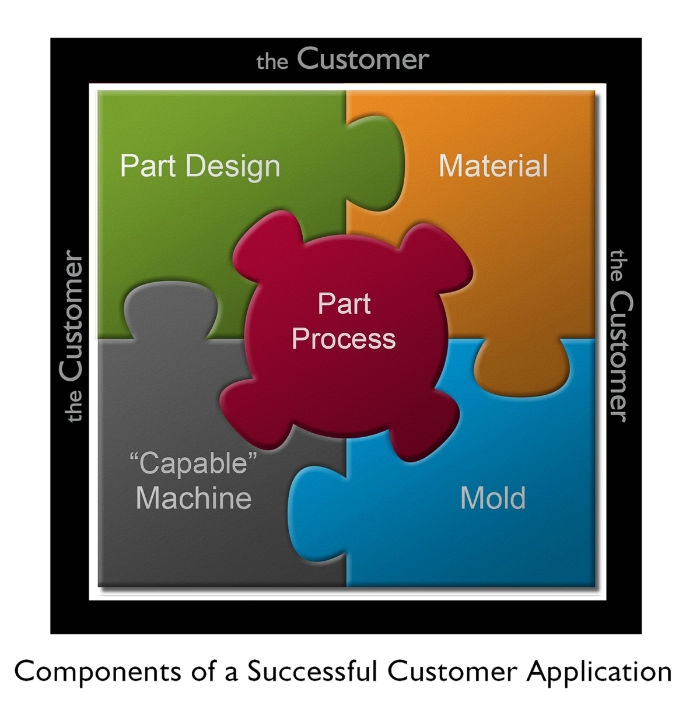
Figure 1: Success starts at the beginning: Components of a successful customer application need representation from all of the respective/responsible team members (end customer, design, quality, assembly/automation, engineering, molding/manufacturing, etc.). Successful design for sustainability (DFS) is based on understanding the entire process—from design to the end user.
Upon completion of this joint team training, communication between the silos improves through the use of similar language and systematic problem solving “from the plastics point of view.” In many cases, simple “why” questions are then sufficient to address areas of concern (e.g., “Why did you gate into that thin section?” “Why did you add those ribs?” “Why are you changing the material?”)
With this newfound education in place, facilitating these types of discussions and addressing these questions promotes collaborative manufacturing. Responses to the previous question may be surprising. A team member might hear, “We copied the feature from the other design,” or “We are not sure we need to have all those ribs,” or “It worked at tryout, but we can’t make parts in the home press.” Typically, these responses are due to a lack of knowledge at the OEM/brand owner level. Designs can be made in a vacuum that impact the supplier’s ability to execute a program successfully, especially if the design is “frozen.” Again, collaboration at all levels is critical.
With the loss of internal expertise and knowhow, there is a recognized need to be able to reach out to third-party resources to facilitate a robust solution and avoid the skepticism and finger pointing that paralyzes the development process. Whether working on the optimization of an existing design or a next-generation launch, reviewing the aforementioned elements of molding helps identify what level of collaboration is necessary, if any.
In addition, having the team benchmark the current state versus desired (future) state of a project, a value stream map of potential obstacles (e.g., resources, costs, quality) can be created, which is an effective method of design for sustainability.
Through this exercise, all stakeholders participated in the collaborative agreement to establish the realistic goals and deliverables. Each takes ownership in executing a sustainable production plan with consistent, repeatable results. Active learning and consulting through project execution with concurrent education and training is a recipe for future sustainable success. This helps the team to realize the cost savings and facilitates more opportunities for future projects.
Execution
Once the CORE team and fundamental educational elements are in place, the enhanced methods to reduce time and waste before introducing a plastic part into manufacturing can be incorporated. Don’t permit shortcuts; use the necessary checks and balances required. Shortcuts in the due diligence process are usually committed if there is a lack of knowledge, experience, and/or understanding.
Having a CORE team comprised of subject matter experts (from the respective companies or departments) can stimulate robust discussion and maintain transparency across the program groups/teams. The diverse experiences that each team member offers can help highlight or identify issues that may not have otherwise been revealed. Through constructive discussion, these concerns can be investigated and vetted out, typically allowing the group to arrive at a more sound, predictive, part/process development method to create a plastic part. The team will have an increased level of confidence that the manufacturing process is capable of producing a product that is well within the upper and lower specification limits, maintained by running within the established control limits.
This approach provides a framework for collaboration between OEM engineering and manufacturing in review of part design versus simulation, educating, optimizing, and assessing the mold development process to avoid uncertainties. Making changes further down the line in the tool launch process is expensive. In almost all cases, concessions are made throughout the process and must be documented as such. Trade-offs that affect others in the process typically cannot be decided unilaterally, but rather, must be resolved collaboratively. Today, more than ever before, OEMs and brand owners are asking for more supplier involvement earlier in the product development phase, especially with plastic components since there is a diminishing pool of internal experts.
Real-World Experience
Following are case studies that illustrate where collaborative manufacturing was successful and, unfortunately, where it wasn’t. Each of the collaborative engagements was entered into with a completed proposal of deliverables that could be tracked to completion. A recurring weekly call was scheduled to keep the team on task. Documentation was maintained on a 24-hour, password-accessible web portal for action items, documentation, and timeline. If one of the team’s external participants completed their part of the process, their access could be removed.
These initiatives were led by RJG’s TZERO team, working with customers on specific applications and concurrently teaching the methods through active learning. Each company is able to use the results as a working case study to apply to future programs. This essentially provides them with a roadmap to facilitate their own internal exercise, engaging additional consulting as needed.
In one example, a customer had a successful design, mold build, and tool launch; however, they did not have the performance specification for airflow included at the onset. The team needs the full picture, including the typical environmental stresses. This would have been caught if the quality department was engaged beyond the dimensional criteria upfront. Unfortunately, a second mold was required to replace the one that failed the performance specifications.
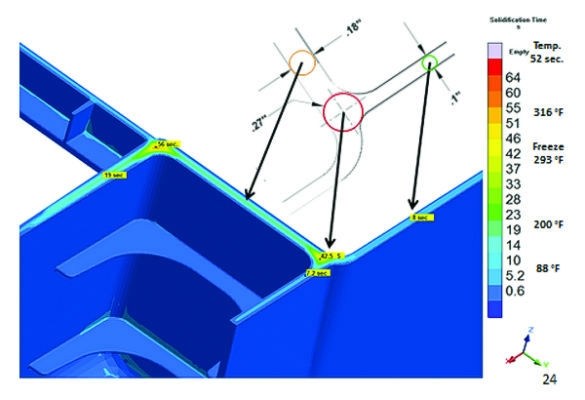
Figure 2: Optimized product design for structural integrity and manufacturability
A successful case study (Figure 2) involved collaboration between part designers and mold designers. During the collaboration call, participants were able to negotiate with the design group to change a feature and eliminate a lifter in a challenging location. The team identified where conductive alloy inserts could be used to reduce the thermal distribution with significant impacts to cycle time and warp. In this situation, the decisions were made collaboratively by the cross-functional team. In many cases, there are trade-offs required when changes are made; understanding these trade-offs up front will allow for strategic decisions later. Figure 2 is a classic example of strategic decision making for design changes to nominal wall or strategic decisions on how to deal with non-uniform walls and potential defects.
Another application involved an OEM’s legacy part that was susceptible to random failure in the field and product return. The OEM wanted to perform their due diligence before making a next-generation tool. Through their own investigation, they identified residual molded-in stress in the part that precipitated cracking failures during use in the field. They wanted to confirm their findings and, based on the components of a successful application, began their analysis.
The OEM, Teleflex Medical (Vascular Division), enlisted the internal and external stakeholders who would be involved in the new tool build—mold maker, hot runner supplier, and RJG’s TZERO team, as well as internal members (Warren Rohde, technical director; DJ Brown, group leader; Harry Koshulsky, molding engineer; and Todd Hilbert, mold designer). The OEM owned the part design, custom material spec., and was the captive molder. The team’s goal was to collectively review the current and desired states, sharing the lessons learned from the previous two tools.
Brown stated, “The team took a disciplined, systematic approach; RJG simplified everything down into the basic elements for easier comprehension by all team members.”
The team exercised multiple iterations of part design and process simulation to arrive at a level of understanding to carry it through the tool build to production. Through the use of three different seats of simulation software (Moldex3D, SigmaSoft, and Moldflow), the team was able to identify and confirm the areas of concerns and demonstrate from where the stress was coming, correlating it to their existing molded results. All aspects of the part and mold design were reviewed to ensure everyone was in agreement with the expected results based on the optimized part design, mold design (cooling), and hot runner considerations to deliver a consistent melt. Collaboration enabled the team to use its collective knowledge and available technology to identify a solution to manufacture parts with the best quality possible and minimize part failures. Since the OEM was involved at the front end, the appropriate part design modifications could be made that still achieved the required fit, form, and function in the field environment for the desired use of the end product.
Conclusion
A collaborative manufacturing approach requires significant effort at the start of a project, but once the team is appropriately informed, the overall effort becomes easier and has a direct effect on profitability through speed to market. Discipline, commitment, and development are necessary to create a performance-based cultural norm. Failure to invest time in collaboration at the beginning often results in delays later in the project schedule due to “redos” while trying to meet deadlines and budget. Teams in this situation sometimes fall into the unacceptable “good enough” mentality. Learn how to ask the right questions—measure, document, assess, modify, and decide.
Individuals and companies that can define CORE teams and execute collaboration effectively (put it into practice) know and actualize the benefits, both in robust product development and profitability. This ultimately leads to long-term sustainability.
Documentation of the team’s execution for each program provides a reference model for future projects. This must always include a “lessons learned” event to close the loop—Were the deliverables established at the beginning achieved? Sometimes, the project teams are capable of adding new deliverables (outside the box) as a result of the diverse backgrounds of each team member.
The value of the team outweighs the individual. By investing in a culture of cross-trained teams, organizations will build momentum in numbers. Therefore, any changes within the human resources of the organization will have a limited impact. The responsibility is distributed across the team and does not fall on any one person’s shoulders.
Collaborative manufacturing drives the right behaviors that support the sustainability of a product and, ultimately, the company.
Matt Therrien is the northeast regional manager for RJG with 28 years of experience focusing on promoting customer success. Strategies include a strong consultative approach, with an emphasis on driving results and developing innovative solutions to a broad range of customer concerns, as well as client education. Therrien has completed the RJG Master Molder Certification Program. He is a BSME graduate of the University of Massachusetts, Amherst, and his experience is drawn from technical and commercial management positions at the global companies Nypro, Husky Injection Molding Systems, MoldMasters Inc., and UPG/MedPlast—where he has implemented successful business models across all market segments, the last 15 years focused on medical device/assembly manufacturing. He can be reached at matt.therrien@rjginc.com.
With specific regard to plastic molded components in a medical device assembly, there are effective risk management approaches that the OEM and outsourcing suppliers can leverage to minimize the probability of recalls. Having an increased level of confidence in the molded components of an assembly provides value by enabling the OEM to focus on the overall performance of the medical device.
As it relates to “cost of quality”—the supply chain’s ability to deliver good parts into the product stream with minimal variation at a cost-effective price—a major task for procurement is to find the value match, not just shop for “piece part price.” With each new project, whether a next-generation device or an enhancement to a legacy product, what’s most important needs to be identified. Further, how best to accomplish the following tasks needs to be considered:
- Develop sustainable parts/products
- Lower cost
- Provide for flexibility (increased manufacturing options)
- Reduce time
- Reduce resources
- Meet the financial analysis
Technical collaboration in business requires the participating companies to become active learning organizations that employ a performance culture that promotes ongoing learning by putting the methods into practice and provides opportunities for growth. To get started, a CORE team must be established, background knowledge developed, and the practical execution of the methods learned.
Identifying the Core Team
The CORE team consists of a champion/coach from each company or department (internal and external stakeholders) who are responsible for transferring learned knowledge to support the common goal of finding a feasible solution to a problem or opportunity through data-driven decisions. It is a best practice approach to transfer knowledge from training and implementation through execution, making the team deep and wide.
The team is responsible for its performance and communication back to each respective department as resources are needed to satisfy the project’s established/approved process flow map.
Not every member of the CORE team is an expert, but the strategy drives the right behavior for the group. Every stakeholder through which the part/device passes during its development/production cycle must have a representative on the team at the beginning. This could include, but is not limited to, the end customer, engineering, quality, assembly and/or automation, molding, manufacturing, and even key members of the supply chain.
Collaboration begins when an honest assessment can be performed to identify strengths and weaknesses. From that analysis, organizations can learn and apply a systematic approach that can be customized for each project, developing a flexible process flow map and establishing an effective method for checks and balances.
Not all projects require full collaboration. By performing a data-driven risk assessment, one can accurately understand the desired deliverables of a project. Evaluation of the risk assessment results (based on a defined scale, considering multiple categories) determines how much collaboration is needed. Assigning a measurement allows organizations to know what’s required and facilitate proper allocation of resources.
Throughout a project, each resource team might have a varying percentage of participation required, freeing them up to perform other job responsibilities and functions.
Building the Knowledge Base
When the investment in individual training is applied and put into practice to educate the cross-functional CORE teams that are established for each project, the core competency learning/experience can be optimized across the entire organization. By no longer working in silos, these teams eliminate the “pass the baton” mentality. Certain elements of CORE teams can be taught and put into practice to enable this to become a reality. It doesn’t happen overnight, but team members will learn how to develop a roadmap, executable plan, and document results for each project.
In many cases, these silos (or geographic distances) are encountered between functional groups. “Throwing it over the wall” to the next department is not a strategy for success. Rather, cross functional education and training with these departments (internal and external) increases communication and data-driven decision making, improving the process flow, understanding the constraints, and documenting the concessions. The first step is an understanding of part/product development “from the plastics point of view,” accomplished through concurrent project execution and method training for future success.
For plastic components in a device assembly, understanding the fundamental principles of injection molding helps the entire CORE team understand the elements and processes required to produce the part.
- Heat—How the plastic is prepared for the next shot (e.g., melted, recovery time, residence time, barrel capacity)
- Flow—How the plastic is introduced into the cavity (e.g., alignment of polymers, shear rates, speeds, consistency)
- Pressure—How the plastic is compressed into the cavity and for how long
- Cooling—How the BTUs are dispersed out of the part and into the mold through the cooling channels (designs and alloys too)

Figure 1: Success starts at the beginning: Components of a successful customer application need representation from all of the respective/responsible team members (end customer, design, quality, assembly/automation, engineering, molding/manufacturing, etc.). Successful design for sustainability (DFS) is based on understanding the entire process—from design to the end user.
Upon completion of this joint team training, communication between the silos improves through the use of similar language and systematic problem solving “from the plastics point of view.” In many cases, simple “why” questions are then sufficient to address areas of concern (e.g., “Why did you gate into that thin section?” “Why did you add those ribs?” “Why are you changing the material?”)
With this newfound education in place, facilitating these types of discussions and addressing these questions promotes collaborative manufacturing. Responses to the previous question may be surprising. A team member might hear, “We copied the feature from the other design,” or “We are not sure we need to have all those ribs,” or “It worked at tryout, but we can’t make parts in the home press.” Typically, these responses are due to a lack of knowledge at the OEM/brand owner level. Designs can be made in a vacuum that impact the supplier’s ability to execute a program successfully, especially if the design is “frozen.” Again, collaboration at all levels is critical.
With the loss of internal expertise and knowhow, there is a recognized need to be able to reach out to third-party resources to facilitate a robust solution and avoid the skepticism and finger pointing that paralyzes the development process. Whether working on the optimization of an existing design or a next-generation launch, reviewing the aforementioned elements of molding helps identify what level of collaboration is necessary, if any.
In addition, having the team benchmark the current state versus desired (future) state of a project, a value stream map of potential obstacles (e.g., resources, costs, quality) can be created, which is an effective method of design for sustainability.
Through this exercise, all stakeholders participated in the collaborative agreement to establish the realistic goals and deliverables. Each takes ownership in executing a sustainable production plan with consistent, repeatable results. Active learning and consulting through project execution with concurrent education and training is a recipe for future sustainable success. This helps the team to realize the cost savings and facilitates more opportunities for future projects.
Execution
Once the CORE team and fundamental educational elements are in place, the enhanced methods to reduce time and waste before introducing a plastic part into manufacturing can be incorporated. Don’t permit shortcuts; use the necessary checks and balances required. Shortcuts in the due diligence process are usually committed if there is a lack of knowledge, experience, and/or understanding.
Having a CORE team comprised of subject matter experts (from the respective companies or departments) can stimulate robust discussion and maintain transparency across the program groups/teams. The diverse experiences that each team member offers can help highlight or identify issues that may not have otherwise been revealed. Through constructive discussion, these concerns can be investigated and vetted out, typically allowing the group to arrive at a more sound, predictive, part/process development method to create a plastic part. The team will have an increased level of confidence that the manufacturing process is capable of producing a product that is well within the upper and lower specification limits, maintained by running within the established control limits.
This approach provides a framework for collaboration between OEM engineering and manufacturing in review of part design versus simulation, educating, optimizing, and assessing the mold development process to avoid uncertainties. Making changes further down the line in the tool launch process is expensive. In almost all cases, concessions are made throughout the process and must be documented as such. Trade-offs that affect others in the process typically cannot be decided unilaterally, but rather, must be resolved collaboratively. Today, more than ever before, OEMs and brand owners are asking for more supplier involvement earlier in the product development phase, especially with plastic components since there is a diminishing pool of internal experts.
Real-World Experience
Following are case studies that illustrate where collaborative manufacturing was successful and, unfortunately, where it wasn’t. Each of the collaborative engagements was entered into with a completed proposal of deliverables that could be tracked to completion. A recurring weekly call was scheduled to keep the team on task. Documentation was maintained on a 24-hour, password-accessible web portal for action items, documentation, and timeline. If one of the team’s external participants completed their part of the process, their access could be removed.
These initiatives were led by RJG’s TZERO team, working with customers on specific applications and concurrently teaching the methods through active learning. Each company is able to use the results as a working case study to apply to future programs. This essentially provides them with a roadmap to facilitate their own internal exercise, engaging additional consulting as needed.
In one example, a customer had a successful design, mold build, and tool launch; however, they did not have the performance specification for airflow included at the onset. The team needs the full picture, including the typical environmental stresses. This would have been caught if the quality department was engaged beyond the dimensional criteria upfront. Unfortunately, a second mold was required to replace the one that failed the performance specifications.

Figure 2: Optimized product design for structural integrity and manufacturability
A successful case study (Figure 2) involved collaboration between part designers and mold designers. During the collaboration call, participants were able to negotiate with the design group to change a feature and eliminate a lifter in a challenging location. The team identified where conductive alloy inserts could be used to reduce the thermal distribution with significant impacts to cycle time and warp. In this situation, the decisions were made collaboratively by the cross-functional team. In many cases, there are trade-offs required when changes are made; understanding these trade-offs up front will allow for strategic decisions later. Figure 2 is a classic example of strategic decision making for design changes to nominal wall or strategic decisions on how to deal with non-uniform walls and potential defects.
Another application involved an OEM’s legacy part that was susceptible to random failure in the field and product return. The OEM wanted to perform their due diligence before making a next-generation tool. Through their own investigation, they identified residual molded-in stress in the part that precipitated cracking failures during use in the field. They wanted to confirm their findings and, based on the components of a successful application, began their analysis.
The OEM, Teleflex Medical (Vascular Division), enlisted the internal and external stakeholders who would be involved in the new tool build—mold maker, hot runner supplier, and RJG’s TZERO team, as well as internal members (Warren Rohde, technical director; DJ Brown, group leader; Harry Koshulsky, molding engineer; and Todd Hilbert, mold designer). The OEM owned the part design, custom material spec., and was the captive molder. The team’s goal was to collectively review the current and desired states, sharing the lessons learned from the previous two tools.
Brown stated, “The team took a disciplined, systematic approach; RJG simplified everything down into the basic elements for easier comprehension by all team members.”
The team exercised multiple iterations of part design and process simulation to arrive at a level of understanding to carry it through the tool build to production. Through the use of three different seats of simulation software (Moldex3D, SigmaSoft, and Moldflow), the team was able to identify and confirm the areas of concerns and demonstrate from where the stress was coming, correlating it to their existing molded results. All aspects of the part and mold design were reviewed to ensure everyone was in agreement with the expected results based on the optimized part design, mold design (cooling), and hot runner considerations to deliver a consistent melt. Collaboration enabled the team to use its collective knowledge and available technology to identify a solution to manufacture parts with the best quality possible and minimize part failures. Since the OEM was involved at the front end, the appropriate part design modifications could be made that still achieved the required fit, form, and function in the field environment for the desired use of the end product.
Conclusion
A collaborative manufacturing approach requires significant effort at the start of a project, but once the team is appropriately informed, the overall effort becomes easier and has a direct effect on profitability through speed to market. Discipline, commitment, and development are necessary to create a performance-based cultural norm. Failure to invest time in collaboration at the beginning often results in delays later in the project schedule due to “redos” while trying to meet deadlines and budget. Teams in this situation sometimes fall into the unacceptable “good enough” mentality. Learn how to ask the right questions—measure, document, assess, modify, and decide.
Individuals and companies that can define CORE teams and execute collaboration effectively (put it into practice) know and actualize the benefits, both in robust product development and profitability. This ultimately leads to long-term sustainability.
Documentation of the team’s execution for each program provides a reference model for future projects. This must always include a “lessons learned” event to close the loop—Were the deliverables established at the beginning achieved? Sometimes, the project teams are capable of adding new deliverables (outside the box) as a result of the diverse backgrounds of each team member.
The value of the team outweighs the individual. By investing in a culture of cross-trained teams, organizations will build momentum in numbers. Therefore, any changes within the human resources of the organization will have a limited impact. The responsibility is distributed across the team and does not fall on any one person’s shoulders.
Collaborative manufacturing drives the right behaviors that support the sustainability of a product and, ultimately, the company.
Matt Therrien is the northeast regional manager for RJG with 28 years of experience focusing on promoting customer success. Strategies include a strong consultative approach, with an emphasis on driving results and developing innovative solutions to a broad range of customer concerns, as well as client education. Therrien has completed the RJG Master Molder Certification Program. He is a BSME graduate of the University of Massachusetts, Amherst, and his experience is drawn from technical and commercial management positions at the global companies Nypro, Husky Injection Molding Systems, MoldMasters Inc., and UPG/MedPlast—where he has implemented successful business models across all market segments, the last 15 years focused on medical device/assembly manufacturing. He can be reached at matt.therrien@rjginc.com.




























