Mark Crawford, Contributing Writer04.03.17
Everybody wants tubing—it’s a strong and steady business, thanks to increased demand for minimally invasive surgery procedures and the use of catheters as delivery systems (for example, ablation and micro implants). Medical device manufacturers (MDMs) and their tubing partners continue to advance tubing functionality, such as the number of lumens, deflection capability, and steerability. According to Markets and Markets, a research firm, the global medical tubing market is expected to grow from $3.73 billion (2015) to $5.85 billion by 2021.1
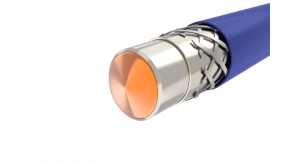
Hundreds of uses exist for medical tubing, each one calling for highly specific physical characteristics. As devices get smaller and more complex, MDMs rely on a greater variety of shapes and sizes of tubing. Device designers especially focus on increased functional capability, such as lumens with non-symmetric cross sections, surface modifications, and the integration of active additives, including antimicrobial agents or drugs. Raw materials are constantly being engineered in new ways to enhance mechanical and biologic characteristics. Popular requests for tubing are custom bump tubing, co-extrusions, micro-extrusions, multi-lumen tubing, profile extrusions, braid-reinforced extrusions, and coated wires. Outside diameters (OD) for tubing can range from about 0.004 inches to 0.45 inches (0.1 mm to 10 mm) or more, with multi-lumen extrusions as small as 0.012 inches (0.305 mm).
Increased demand for smaller diameters, thinner walls, and more complex lumens constantly push the limits of existing extrusion and production technology. One of the greatest design challenges tubing manufacturers face is the request for a smaller-diameter tube that will accept a greater number of lumens packed inside—for example, six lumens in a 3 Fr catheter tube. In response to these exacting design demands, tubing extruders must find new, often proprietary ways to make tubing products. It comes down to seeing how far science can go—and then finding ways to push science a little further.
“Tubing is one of the more exciting and faster-growing areas in the medical device industry,” said Steven Burdorf, president of Catheter & Medical Design Inc., a St. Paul, Minn.-based provider of cardiovascular, neurosurgical, and laparoscopic tubing. “The decision-making and detailed knowledge required to meet evolving customer expectations is challenging and rewarding. As medical device applications expand, the properties of the tubing such as flexibility, lubricity, clarity, kink resistance, and the ability to hold tight tolerances are all critical for determining the ideal design for a particular medical application.”
In fact, added Tim Steele, founder and CEO of Microspec Corporation, a Peterborough, N.H.-based custom extruder of medical tubing, “it can be so challenging that in integrating new technologies, desired results can be so theoretical they may not even be indicated on the engineering/quality specification used in making the part.”
Yet extruders continue to find ways to coax more out of their high-performance polymer materials and sophisticated extrusion machines when customers come calling with the next design that challenges innovative thinking.
“The tubing market is quite strong,” said Marcia Coulson, president of Eldon James Corporation, a Denver, Colo.-based manufacturer of tubing, hose fittings, and other accessories. “We find that we are talking with customers every day about innovative new tubing requests, or how they can use our current product lines.”
What OEMs Want
MDMs want smaller diameters and thinner walls. But their wish list isn’t just about dimensions—they also strive for increased functionality in their tubing, such as engineered materials, multi-durometer tubing, and multilayer tubing. As multi-lumen tubing increases its capacity to have a greater number of lumen sizes within a single shaft, the concentricity and wall thickness of each lumen become more difficult to manufacture. Micro-catheter designs require thin wall thicknesses that are still strong enough to maintain accurate pressures and flow mechanics. And of course, MDMs want all these features at the lowest possible cost.
Because they do not have the necessary engineering staff and/or expertise to manage or handle everything it takes to develop medical devices and bring them to market, OEMs outsource much of this development work to experts within their supply chain—including tubing vendors. “As a result, there is more interest by OEMs in ‘one-stop-shop’ contract manufacturing that not only does the extrusion, but also handles downstream secondary operations, including full assemblies,” said Tom Doering, global market segment manager for Lubrizol LifeSciences, a Cleveland, Ohio-based contract manufacturer that provides polymer tubing solutions to the medical device industry.
Coulson agreed. “OEMs are looking for tubing suppliers who not only extrude, but also cut to length and assemble fittings, clamps, valves, and quick disconnects,” she added.
Developing “one-stop shop” partnerships with key suppliers that share their specialized knowledge and expertise streamlines the entire production process. It also provides more control over processes, validation, production costs, communications, and delivery—thereby reducing operational costs and rework and delivering products into the marketplace faster.
“Both large and small OEMs are looking for partnerships and engineers dedicated to achieving delivery of complex designs and, once in production, the need to provide cost-out in order to retain their business and/or be awarded new business,” said Burdorf. “Smaller OEMs and startups usually have designs, but are looking for quick turnaround times in smaller quantities.”
Latest Trends in Tubing
A big trend in tubing is utilizing more lumens for multiple functions, which reduces the number of tubes required for a medical procedure, especially those that are performed in a restricted space (for example, cardiovascular). Thinner-walled tubes can accept a greater number of lumens inside the tubes. Achieving super-thin walls pushes the limits of technology and repeatability—however, the wall must also be strong enough to meet pressure testing and manufacturing repeatability requirements.
“The challenges we face are thinness of inner wall lining and maintaining consistency throughout the tube, as well as maintaining lumen alignment,” said Burdoff. “The thinner the inner wall lining, the more lumens can be accommodated. Depending on tolerance requirements, the inner wall lining can be a few thousandths of an inch thick. The majority of lumen requests we receive are in the two to four range, although we have gone up to seven.”
Tubing manufacturers are seeing more requests for tighter tolerances and thinner wall thicknesses. This trend is driven by designers who need to fit more into less space, without having to worry about tolerance stack-up issues. “We make small tubing with walls as thin as 0.0005 inches,” said Duane Dunn, president of Dunn Industries Inc., a Manchester, N.H.-based custom extruder. “It is important to be careful with handling—with walls that thin, the tubing must be handled very delicately so it does not kink.”
Bucking the miniaturization trend is the growing need for large diameter shafts—up to 35 F—that can maintain dimensional stability and concentricity. Some OEMs are asking for flexible tubing manufactured in sizes that exceed one inch inside diameter (ID). This larger tubing is commonly used with large catheters for heart valve replacements that offer drainage through tubing lumens, while still allowing fluids and medications to be administered through other lumens within the same tube. For example, a delivery system for a trans-catheter heart valve entering through the femoral artery may require tubing with an ID up to 24 F (0.315 in. or 8 mm) or more.
“Some of our clients are asking us to make very large OD and ID tubing with thin walls, 10 mm plus in size,” said Dunn.
When they can, MDMs prefer to use off-the-shelf tubing/samples and quick-turn custom extrusions to minimize development time and costs. “This is what has led LLS/Vesta to develop a rapid extrusion program to handle mainstream tubing requests, with as little as a one-week turnaround time for a custom extrusion,” said Doering.
However, off-the-shelf options become less likely as devices get smaller and more complex, pushing developers toward customized tubing in order to deliver the functionality they expect. Many new surgical or treatment techniques require tubing functions that cannot be attained with off-the-shelf tubing. Examples of increased functionality include adding electrical wires into the wall of the tubing so it can power a device or sensors without needing to run wires down the ID, as in hybrid tubing. Adding reinforcement or providing varying dimensions of the tube along its length also typically require a customized solution. High-pressure tubing that must meet thin wall requirements and withstand high absolute pressures is typically custom made. In extruded parallel tubing, the extruder creates custom parallel tubing products from a wide range of thermoplastic materials. Multi-layer extrusion can manufacture tubing using multiple materials within a single tube, blending key properties from each material to create tubing with highly specific performance specifications.
A strong trend toward customization also exists in the stainless steel tubing market. In the past, device engineers typically requested off-the-shelf catalog tubing because it was available quickly. The downside to this approach was that design compromises often resulted; extra time was also needed to make the mating components work correctly.
“We have seen the industry rapidly shift from trying to make stock tubing work versus viewing the tubing as a design advantage,” said Jeffrey Crane, application engineer for K-Tube Technologies, a Poway, Calif.-based supplier of miniature stainless steel tubing. “A decade ago, most of our tubing sales were from our catalog, but 90 percent of what K-Tube manufactures today is custom tubing.”
Engineers want application-specific physical properties that will give them a competitive advantage. This usually translates into using different alloys, but sometimes it involves pushing capabilities in manufacturing as well.
“Today, device engineers collaborate directly with the provider to design custom tubing that improves device performance,” said Crane. “We tweak dimensions, tighten critical tolerances, improve surface finishes, and manage physical properties like hardness, tensile strength, and stiffness by either altering the manufacturing process or recommending a better alloy.”
For example, he added, “if we are tailoring properties for a catheter-based delivery system, we might recommend tubing with very high yield strengths, and then manufacture concurrent lots in Co-Cr alloys [MP35N, L605] or other precipitation hardened alloys such as 17-7PH or 1RK1, so they can test torquability and pushability using different materials.”
Material Challenges
OEM requests for smaller, thinner, more functional, and customized tubing all, to a large degree, depend on the type of material selected for the tubing. MDMs are increasingly interested in making products from the thousands of highly engineered thermoplastic resins that provide a range of enhanced physical properties. These include strength, flexibility, pushability, radio-opacity, anticoagulation, lubricity, and biostability. Polyether ether-ketone (PEEK) is a light, strong thermoplastic polymer with excellent strength and heat endurance and generates little friction, making it a popular choice for catheter-based applications. Some PEEK products can be extruded with walls as thin as 0.006 in. and tolerances of about ±0.0015 in. ID/OD.
Advanced extrusion materials include polytetrafluoroethylene (PTFE) tubing. PTFE provides high temperature and chemical resistance, tensile strength, and a very low coefficient of friction—making it ideal for catheter sheathing, wire coating, stent delivery, endotracheal devices, or any applications that require a material with a very low coefficient of friction, high abrasion resistance, and insulation properties. It can also be co-extruded with barium sulfate stripes, which are widely used in IV catheter tubing.
Even though PVC is still the most popular material for tubing, more companies are moving away from this material due to concerns that it may interfere with drug dosing efficacy and, as a result, eventually be severely restricted or banned from medical devices for toxicity concerns. In response, especially for high-volume PVC tubing demands, companies are looking for PVC substitutes such as polypropylene blends and thermoplastics elastomers (TPEs). A variety of TPE compounds provide the same clarity, haptics, physical properties, and kink- and clamp-resistance of PVC and are relatively easy to extrude; they also show enhanced gamma stability and flexibility. Compared to silicone, TPEs are easier to extrude and don’t require a curing step.
“We are finding that customers who might have been using silicone before can now consider TPEs,” said Coulson. “Many customers have found they can save money by using TPE for pumping applications. If high temperature is not required, TPE is a great alternative to silicone in many applications.”
Bioabsorbable polymers such as poly-L-lactic acid and poly (lactic-co-glycolic acid) are rapidly gaining traction and will have a big impact on material selection for medical tubing. These materials are expensive, however, so tubing suppliers must fully understand their physical characteristics and how they are impacted or degraded by the extrusion process (for example, prolonged residence time). Concentricity—uniform wall thickness—of the tubing is also highly critical. Bioresorbable tubing is designed with a specific degradation profile that allows the materials to perform in the body for a fixed period of time before being resorbed.
Material producers continue to release new hybrid and blended materials with various additives that enhance characteristics such as strength, heat, chemical resistance, flexibility, and durometer. Many new materials are also highly heat- or moisture-sensitive and require additional care in handling and processing to maintain material integrity.
Perhaps the biggest challenge is combining materials—for example, combining the physical properties of silicone and thermoplastics through a co-extrusion process. The outer silicone material provides flexibility and strong bonding characteristics with fittings, while the thermoplastic lining provides barrier properties. One of the most challenging aspects of co-extrusion is creating a strong bond between the silicone and the variety of substrates that OEMs request for multilayered designs. Sometimes a primer or surface preparation using plasma or corona etching is needed to enhance bonding.
Moving Forward
Extruders are becoming accustomed to the nonstop pace of their business—especially the urgency for higher performance, faster turnaround, longer lifecycle, reduced cost, and inspection/validation. MDMs count on their extruders to take the lead in finding the right materials to deliver the proper physical characteristics for their tubing, as well as having the technical ability to extrude extremely complex multi-lumen structures and unique profiles. MDMs are also intensely focused on functionality, speed to market, and cost. As a result, tubing continues to push the technology envelope as more advanced materials and innovative technologies are developed. OEMs and their suppliers must stay on the leading edge of technology, material science, and best practices to meet these evolving manufacturing challenges—and reduce risk.
“We work with device companies every day to manage risk, either through our quality system, regulatory experience, or by simply running multiple test lots for validation purposes,” said Crane. “The FDA is diving deeper into the supply chain, which gives us an advantage over those importing steel from abroad and then reselling under a generic certificate of compliance. The process of focusing early on critical ‘raw material’ design has helped our customers manage risk and improved patient outcomes.”
“We have so many great materials that have been introduced to the market,” added Coulson. “I think manufacturers can be a limiting factor because they don’t always have time or resources available to evaluate new materials.”
“The improvement we will see in the future comes from us working together to push the limits of what is possible,” continued Crane. “It’s difficult for a supplier to know what the industry wants without getting direct feedback on the end use and application. We encourage customers to keep asking for the optimum solution, instead of settling for what’s currently available.”
This approach works best when the MDM develops strong relationships with their key partners, early in the concept/design stage. Good communication is essential, especially as tubing pushes the limits of science. “Depending on the technical complexity of the part, up-front discussions could be extensive between the engineering and manufacturing teams,” said Steele. “Defects can be avoided through good planning and effective communication.”
Bear in mind the first extrusion attempts typically fall short of specifications. Custom extrusion companies encounter challenging parts regularly and working them through until the part is acceptable to the customer requires understanding on both sides. “This sometimes means more than several extrusion runs, openness, and being willing to change a specification to match parts that meet performance requirements, but not all aspects of the original specification,” added Steele.
Once production starts and the products are proven to work, the customer needs that production process to be validated. According to Steele, both the customer and extruder need to mutually understand that results obtained in the operational qualification/performance qualification extrusion runs may show capability, but the scope of the validation must address that the data in the report represents only four separate extrusion runs, and it should be assumed during production that all variables in the process have not been defined. “Addressing this issue in the validation report is critical to future success in production,” he said.
The development of an advanced, custom medical extrusion can be very complicated. The key to success, outside of the extruder’s expertise, is communication. “Effective communication is built on the openness of both parties, good listening, and mutual understanding of the issues,” concluded Steele. “They must also understand that in validation there are still future variables that may impact the production process and need to be accommodated by creating latitude in the process limits, based upon the limited data used in the validation report.”
References
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books. Contact him at mark.crawford@charter.net.
Hundreds of uses exist for medical tubing, each one calling for highly specific physical characteristics. As devices get smaller and more complex, MDMs rely on a greater variety of shapes and sizes of tubing. Device designers especially focus on increased functional capability, such as lumens with non-symmetric cross sections, surface modifications, and the integration of active additives, including antimicrobial agents or drugs. Raw materials are constantly being engineered in new ways to enhance mechanical and biologic characteristics. Popular requests for tubing are custom bump tubing, co-extrusions, micro-extrusions, multi-lumen tubing, profile extrusions, braid-reinforced extrusions, and coated wires. Outside diameters (OD) for tubing can range from about 0.004 inches to 0.45 inches (0.1 mm to 10 mm) or more, with multi-lumen extrusions as small as 0.012 inches (0.305 mm).
Increased demand for smaller diameters, thinner walls, and more complex lumens constantly push the limits of existing extrusion and production technology. One of the greatest design challenges tubing manufacturers face is the request for a smaller-diameter tube that will accept a greater number of lumens packed inside—for example, six lumens in a 3 Fr catheter tube. In response to these exacting design demands, tubing extruders must find new, often proprietary ways to make tubing products. It comes down to seeing how far science can go—and then finding ways to push science a little further.
“Tubing is one of the more exciting and faster-growing areas in the medical device industry,” said Steven Burdorf, president of Catheter & Medical Design Inc., a St. Paul, Minn.-based provider of cardiovascular, neurosurgical, and laparoscopic tubing. “The decision-making and detailed knowledge required to meet evolving customer expectations is challenging and rewarding. As medical device applications expand, the properties of the tubing such as flexibility, lubricity, clarity, kink resistance, and the ability to hold tight tolerances are all critical for determining the ideal design for a particular medical application.”
In fact, added Tim Steele, founder and CEO of Microspec Corporation, a Peterborough, N.H.-based custom extruder of medical tubing, “it can be so challenging that in integrating new technologies, desired results can be so theoretical they may not even be indicated on the engineering/quality specification used in making the part.”
Yet extruders continue to find ways to coax more out of their high-performance polymer materials and sophisticated extrusion machines when customers come calling with the next design that challenges innovative thinking.
“The tubing market is quite strong,” said Marcia Coulson, president of Eldon James Corporation, a Denver, Colo.-based manufacturer of tubing, hose fittings, and other accessories. “We find that we are talking with customers every day about innovative new tubing requests, or how they can use our current product lines.”
What OEMs Want
MDMs want smaller diameters and thinner walls. But their wish list isn’t just about dimensions—they also strive for increased functionality in their tubing, such as engineered materials, multi-durometer tubing, and multilayer tubing. As multi-lumen tubing increases its capacity to have a greater number of lumen sizes within a single shaft, the concentricity and wall thickness of each lumen become more difficult to manufacture. Micro-catheter designs require thin wall thicknesses that are still strong enough to maintain accurate pressures and flow mechanics. And of course, MDMs want all these features at the lowest possible cost.
Because they do not have the necessary engineering staff and/or expertise to manage or handle everything it takes to develop medical devices and bring them to market, OEMs outsource much of this development work to experts within their supply chain—including tubing vendors. “As a result, there is more interest by OEMs in ‘one-stop-shop’ contract manufacturing that not only does the extrusion, but also handles downstream secondary operations, including full assemblies,” said Tom Doering, global market segment manager for Lubrizol LifeSciences, a Cleveland, Ohio-based contract manufacturer that provides polymer tubing solutions to the medical device industry.
Coulson agreed. “OEMs are looking for tubing suppliers who not only extrude, but also cut to length and assemble fittings, clamps, valves, and quick disconnects,” she added.
Developing “one-stop shop” partnerships with key suppliers that share their specialized knowledge and expertise streamlines the entire production process. It also provides more control over processes, validation, production costs, communications, and delivery—thereby reducing operational costs and rework and delivering products into the marketplace faster.
“Both large and small OEMs are looking for partnerships and engineers dedicated to achieving delivery of complex designs and, once in production, the need to provide cost-out in order to retain their business and/or be awarded new business,” said Burdorf. “Smaller OEMs and startups usually have designs, but are looking for quick turnaround times in smaller quantities.”
Latest Trends in Tubing
A big trend in tubing is utilizing more lumens for multiple functions, which reduces the number of tubes required for a medical procedure, especially those that are performed in a restricted space (for example, cardiovascular). Thinner-walled tubes can accept a greater number of lumens inside the tubes. Achieving super-thin walls pushes the limits of technology and repeatability—however, the wall must also be strong enough to meet pressure testing and manufacturing repeatability requirements.
“The challenges we face are thinness of inner wall lining and maintaining consistency throughout the tube, as well as maintaining lumen alignment,” said Burdoff. “The thinner the inner wall lining, the more lumens can be accommodated. Depending on tolerance requirements, the inner wall lining can be a few thousandths of an inch thick. The majority of lumen requests we receive are in the two to four range, although we have gone up to seven.”
Tubing manufacturers are seeing more requests for tighter tolerances and thinner wall thicknesses. This trend is driven by designers who need to fit more into less space, without having to worry about tolerance stack-up issues. “We make small tubing with walls as thin as 0.0005 inches,” said Duane Dunn, president of Dunn Industries Inc., a Manchester, N.H.-based custom extruder. “It is important to be careful with handling—with walls that thin, the tubing must be handled very delicately so it does not kink.”
Bucking the miniaturization trend is the growing need for large diameter shafts—up to 35 F—that can maintain dimensional stability and concentricity. Some OEMs are asking for flexible tubing manufactured in sizes that exceed one inch inside diameter (ID). This larger tubing is commonly used with large catheters for heart valve replacements that offer drainage through tubing lumens, while still allowing fluids and medications to be administered through other lumens within the same tube. For example, a delivery system for a trans-catheter heart valve entering through the femoral artery may require tubing with an ID up to 24 F (0.315 in. or 8 mm) or more.
“Some of our clients are asking us to make very large OD and ID tubing with thin walls, 10 mm plus in size,” said Dunn.
When they can, MDMs prefer to use off-the-shelf tubing/samples and quick-turn custom extrusions to minimize development time and costs. “This is what has led LLS/Vesta to develop a rapid extrusion program to handle mainstream tubing requests, with as little as a one-week turnaround time for a custom extrusion,” said Doering.
However, off-the-shelf options become less likely as devices get smaller and more complex, pushing developers toward customized tubing in order to deliver the functionality they expect. Many new surgical or treatment techniques require tubing functions that cannot be attained with off-the-shelf tubing. Examples of increased functionality include adding electrical wires into the wall of the tubing so it can power a device or sensors without needing to run wires down the ID, as in hybrid tubing. Adding reinforcement or providing varying dimensions of the tube along its length also typically require a customized solution. High-pressure tubing that must meet thin wall requirements and withstand high absolute pressures is typically custom made. In extruded parallel tubing, the extruder creates custom parallel tubing products from a wide range of thermoplastic materials. Multi-layer extrusion can manufacture tubing using multiple materials within a single tube, blending key properties from each material to create tubing with highly specific performance specifications.
A strong trend toward customization also exists in the stainless steel tubing market. In the past, device engineers typically requested off-the-shelf catalog tubing because it was available quickly. The downside to this approach was that design compromises often resulted; extra time was also needed to make the mating components work correctly.
“We have seen the industry rapidly shift from trying to make stock tubing work versus viewing the tubing as a design advantage,” said Jeffrey Crane, application engineer for K-Tube Technologies, a Poway, Calif.-based supplier of miniature stainless steel tubing. “A decade ago, most of our tubing sales were from our catalog, but 90 percent of what K-Tube manufactures today is custom tubing.”
Engineers want application-specific physical properties that will give them a competitive advantage. This usually translates into using different alloys, but sometimes it involves pushing capabilities in manufacturing as well.
“Today, device engineers collaborate directly with the provider to design custom tubing that improves device performance,” said Crane. “We tweak dimensions, tighten critical tolerances, improve surface finishes, and manage physical properties like hardness, tensile strength, and stiffness by either altering the manufacturing process or recommending a better alloy.”
For example, he added, “if we are tailoring properties for a catheter-based delivery system, we might recommend tubing with very high yield strengths, and then manufacture concurrent lots in Co-Cr alloys [MP35N, L605] or other precipitation hardened alloys such as 17-7PH or 1RK1, so they can test torquability and pushability using different materials.”
Material Challenges
OEM requests for smaller, thinner, more functional, and customized tubing all, to a large degree, depend on the type of material selected for the tubing. MDMs are increasingly interested in making products from the thousands of highly engineered thermoplastic resins that provide a range of enhanced physical properties. These include strength, flexibility, pushability, radio-opacity, anticoagulation, lubricity, and biostability. Polyether ether-ketone (PEEK) is a light, strong thermoplastic polymer with excellent strength and heat endurance and generates little friction, making it a popular choice for catheter-based applications. Some PEEK products can be extruded with walls as thin as 0.006 in. and tolerances of about ±0.0015 in. ID/OD.
Advanced extrusion materials include polytetrafluoroethylene (PTFE) tubing. PTFE provides high temperature and chemical resistance, tensile strength, and a very low coefficient of friction—making it ideal for catheter sheathing, wire coating, stent delivery, endotracheal devices, or any applications that require a material with a very low coefficient of friction, high abrasion resistance, and insulation properties. It can also be co-extruded with barium sulfate stripes, which are widely used in IV catheter tubing.
Even though PVC is still the most popular material for tubing, more companies are moving away from this material due to concerns that it may interfere with drug dosing efficacy and, as a result, eventually be severely restricted or banned from medical devices for toxicity concerns. In response, especially for high-volume PVC tubing demands, companies are looking for PVC substitutes such as polypropylene blends and thermoplastics elastomers (TPEs). A variety of TPE compounds provide the same clarity, haptics, physical properties, and kink- and clamp-resistance of PVC and are relatively easy to extrude; they also show enhanced gamma stability and flexibility. Compared to silicone, TPEs are easier to extrude and don’t require a curing step.
“We are finding that customers who might have been using silicone before can now consider TPEs,” said Coulson. “Many customers have found they can save money by using TPE for pumping applications. If high temperature is not required, TPE is a great alternative to silicone in many applications.”
Bioabsorbable polymers such as poly-L-lactic acid and poly (lactic-co-glycolic acid) are rapidly gaining traction and will have a big impact on material selection for medical tubing. These materials are expensive, however, so tubing suppliers must fully understand their physical characteristics and how they are impacted or degraded by the extrusion process (for example, prolonged residence time). Concentricity—uniform wall thickness—of the tubing is also highly critical. Bioresorbable tubing is designed with a specific degradation profile that allows the materials to perform in the body for a fixed period of time before being resorbed.
Material producers continue to release new hybrid and blended materials with various additives that enhance characteristics such as strength, heat, chemical resistance, flexibility, and durometer. Many new materials are also highly heat- or moisture-sensitive and require additional care in handling and processing to maintain material integrity.
Perhaps the biggest challenge is combining materials—for example, combining the physical properties of silicone and thermoplastics through a co-extrusion process. The outer silicone material provides flexibility and strong bonding characteristics with fittings, while the thermoplastic lining provides barrier properties. One of the most challenging aspects of co-extrusion is creating a strong bond between the silicone and the variety of substrates that OEMs request for multilayered designs. Sometimes a primer or surface preparation using plasma or corona etching is needed to enhance bonding.
Moving Forward
Extruders are becoming accustomed to the nonstop pace of their business—especially the urgency for higher performance, faster turnaround, longer lifecycle, reduced cost, and inspection/validation. MDMs count on their extruders to take the lead in finding the right materials to deliver the proper physical characteristics for their tubing, as well as having the technical ability to extrude extremely complex multi-lumen structures and unique profiles. MDMs are also intensely focused on functionality, speed to market, and cost. As a result, tubing continues to push the technology envelope as more advanced materials and innovative technologies are developed. OEMs and their suppliers must stay on the leading edge of technology, material science, and best practices to meet these evolving manufacturing challenges—and reduce risk.
“We work with device companies every day to manage risk, either through our quality system, regulatory experience, or by simply running multiple test lots for validation purposes,” said Crane. “The FDA is diving deeper into the supply chain, which gives us an advantage over those importing steel from abroad and then reselling under a generic certificate of compliance. The process of focusing early on critical ‘raw material’ design has helped our customers manage risk and improved patient outcomes.”
“We have so many great materials that have been introduced to the market,” added Coulson. “I think manufacturers can be a limiting factor because they don’t always have time or resources available to evaluate new materials.”
“The improvement we will see in the future comes from us working together to push the limits of what is possible,” continued Crane. “It’s difficult for a supplier to know what the industry wants without getting direct feedback on the end use and application. We encourage customers to keep asking for the optimum solution, instead of settling for what’s currently available.”
This approach works best when the MDM develops strong relationships with their key partners, early in the concept/design stage. Good communication is essential, especially as tubing pushes the limits of science. “Depending on the technical complexity of the part, up-front discussions could be extensive between the engineering and manufacturing teams,” said Steele. “Defects can be avoided through good planning and effective communication.”
Bear in mind the first extrusion attempts typically fall short of specifications. Custom extrusion companies encounter challenging parts regularly and working them through until the part is acceptable to the customer requires understanding on both sides. “This sometimes means more than several extrusion runs, openness, and being willing to change a specification to match parts that meet performance requirements, but not all aspects of the original specification,” added Steele.
Once production starts and the products are proven to work, the customer needs that production process to be validated. According to Steele, both the customer and extruder need to mutually understand that results obtained in the operational qualification/performance qualification extrusion runs may show capability, but the scope of the validation must address that the data in the report represents only four separate extrusion runs, and it should be assumed during production that all variables in the process have not been defined. “Addressing this issue in the validation report is critical to future success in production,” he said.
The development of an advanced, custom medical extrusion can be very complicated. The key to success, outside of the extruder’s expertise, is communication. “Effective communication is built on the openness of both parties, good listening, and mutual understanding of the issues,” concluded Steele. “They must also understand that in validation there are still future variables that may impact the production process and need to be accommodated by creating latitude in the process limits, based upon the limited data used in the validation report.”
References
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books. Contact him at mark.crawford@charter.net.