Michael Barbella, Managing Editor11.08.16
For the record: 2016 has not been a bad year for medtech. But it hasn’t been a remarkably great year, either. Truthfully, it’s been one of those run-of-the-mill, hard-to-define eras, where the days seem to blend together almost in an indistinguishable blur. It’s been a year of routine, of familiarity, as the medical device industry further adapts to fundamental shifts in reimbursement, digital health, payer engagement, and value-based care.
In many respects, 2016 has seemed like a continuation of its predecessor. The lines between medtech, health IT, healthcare services, and even therapeutics blurred more this year with Medtronic plc’s investments in consulting and hospital-managed services, and Johnson & Johnson’s participation in the setup of Verb Surgical, its robotic surgery joint venture with Verily, née Google Life Sciences.
The drive for scale and strength among OEMs continued with Abbott Laboratories’ $25 billion bid for St. Jude Medical Inc., Thermo Fisher Scientific Inc.’s $4.2 billion purchase of FEI Company, Stryker Corp.’s $2.77 billion acquisition of Sage Products LLC, and Medtronic’s $1.1 billion purchase of Heartware International Inc. Likewise, companies also maintained their affinity in 2016 for bolt-on transactions to fill portfolio gaps and divestitures to exit non-core competency areas or shed poorly-performing business units.
Big data and cybersecurity issues lingered well into the year too, as the industry began digesting guidance from the U.S. Food and Drug Administration (FDA) on the use of real-world data, as well as recommendations on mitigating device cybersecurity threats following the 2015 discovery of defensive vulnerabilities in Hospira’s infusion pump systems.
The year, however, was not without its own defining moments, though. The most notable, obviously, was the suspension of the 2.3 percent medical device tax—a longstanding source of contention between industry leaders and Obamacare proponents. There was also the reclassification of transvaginal surgical mesh (from Class II to Class III), the FDA’s second-ever device ban (on powdered surgical gloves), the release of a redesigned (and presumably safer) duodenoscope, and the official coronation of a new FDA commissioner.
For insight into these and other kairos of the past 10 months, take a stroll down memory lane, courtesy of Medical Product Outsourcing.
Theranos’ Terrible, Horrible, No Good Very Bad Year
It wasn’t supposed to turn out this way.

In June, Forbes cut Theranos CEO Elizabeth Holmes’ net worth
from an estimated $4.5 billion to 0.
Elizabeth Holmes had big plans. World-changing plans. The blood testing technology developed by her Silicon Valley startup was going to revolutionize healthcare, in much the same way Apple Inc. transformed the computer industry. “This is about being able to do good,” Holmes told Fortune in 2014. “And it’s about being able to change the healthcare system through what we believe this country does so well, which is innovation and creativity and the ability to conceive of technology that can help solve policy challenges.”
Holmes’ quest to do good turned inexorably bad relatively quickly, though. As her company, Theranos Inc., steadily gained in valuation—eventually reaching $9 billion—clinical pathologists and biochemists became skeptical of the technology the firm developed to measure up to 70 biomarkers from a single finger-prick blood sample. Some experts criticized the lack of peer review for the tests; others lambasted the secrecy surrounding the testing. Exacerbating those concerns was an August 2015 FDA inspection that found uncleared medical devices and objectionable conditions inside Theranos’ facilities.
The company (and Holmes) suffered a more damaging blow the following month after a Wall Street Journal investigation accused Theranos of using its flagship technology for just a small number of tests, relying on conventional devices for most assays, and releasing questionable results to patients.
Now, Theranos’ fairy tale-like existence seems destined for an unhappily-ever-after ending. Since the Journal article appeared last fall, the company has voided tens of thousands of test results, faces federal civil and criminal investigations, and is appealing the revocation of its blood testing license at its Newark, Calif., lab. This year has been particularly challenging for the firm: In January, the Centers for Medicare and Medicaid Services released a scathing inspection report, prompting Walgreens to terminate its three-year partnership with the company and shutter all Theranos lab-testing services at its retail locations.
In June, Forbes cut Holmes’ net worth to zero, based on the assumption that Theranos was worth roughly $800 million—the estimated value of its investment capital. Then in October, the embattled company laid off 340 people (about 43 percent of its staff) as it pulled the plug on its clinical labs and Theranos Wellness centers in California, Arizona, and Texas.
Holmes’ dream was officially dead.
Rather than mourn, however, Holmes set her sights on a new goal: Developing the miniLab, a 95-pound, tabletop-sized diagnostic tool designed to reduce the cost and improve the accessibility of blood tests. “Our ultimate goal is to commercialize miniaturized, automated laboratories capable of small-volume sample testing, with an emphasis on vulnerable patient populations, including oncology, pediatrics, and intensive care,” Holmes wrote in an Oct. 5 blog post on the company’s website. “We have a new executive team leading our work toward obtaining FDA clearances, building commercial partnerships, and pursuing publications in scientific journals. We are fortunate to have supporters and investors who believe deeply in our mission of affordable, less invasive lab testing, and to have the runway to realize our vision.”
Theranos’ new vision is not quite ready for takeoff, though. Less than a week after announcing the pivot plan, a major hedge fund investor sued the company and Holmes for “knowingly and repeatedly” lying to attract $96 million in financing. An Oct. 10 letter to investors from San Francisco, Calif.-based Partner Fund Management LP accused Theranos and its CEO/founder of engaging in securities fraud and other violations by inducing the hedge fund to invest in the startup, according to the Journal and Forbes.
“Among other things, Theranos and its principals knowingly and repeatedly lied that they had developed proprietary technologies that worked, were on the cusp of receiving all necessary regulatory clearances, and approvals, and concealed the truth about the commercial viability of their technologies and methods,” Forbes reported, quoting the letter.
Theranos, in turn, denied the charges, and countered with its own claims: “The suit is without merit, the assertions are baseless, and the plaintiff is engaging in revisionist history,” the company said in an official statement. “Most of the company statements the plaintiff has cited in its suit were made after the time the plaintiff invested, and could not possibly have been the original basis for investment. This wholesale reliance on post-investment statements, therefore, negates the claim that the plaintiff was misled.”
PFM’s lawsuit, filed under seal in Delaware Chancery Court, seeks to recoup the firm’s investment plus damages and costs associated with the claim, the Journal noted.
Although it remains emphatic about its innocence, PFM’s allegations create a headache for Theranos as it attempts to start anew. The lawsuit could potentially prompt other financiers to seek retribution for lost investments, or worse, draw further scrutiny from federal investigators. At the very least, it will likely further sully the company’s (and Holmes’) already tarnished reputation, possibly leading to future problems with investors and government regulators. Such setbacks could eventually threaten Theranos’ existence.
As Forbes’ Matthew Herper opined when Theranos announced its pivot: “The wax on this company’s wings has melted, and it is not clear when Theranos will hit the ground.”
Prepare for impact.
Read more: http://bit.ly/yir1605
The Tax Man Leaveth
The day went largely unnoticed. There were no formal celebrations marking the moment, no proclamations immortalizing the date, and no special gatherings acknowledging the occasion. The day, in fact, was no different than any other that week. Except that it was a Monday. The start of a new workweek. The beginning of a new month.
Monday, Feb. 1 was also the start of a new era for the medtech industry: It was the final medical device excise tax payment deadline.
For two years, anyway. Or perhaps, ever.
Long a thorn in the industry’s side, the 2.3 percent medical device tax was suspended late last year through 2017 as part of a compromise $1.8 trillion spending package that prevented a government shutdown. The suspension capped a long, hard-fought repeal effort by MDMA, the Advanced Medical Technology Association, and other lobbying groups that opposed the levy for its chilling effect on innovation and R&D, as well as its drain on corporate finances. The fight triggered at least five showdowns in the U.S. House of Representatives, two of which featured stand-alone repeal measures, and two involved inclusions in a jobs package and a reconciliation vote.
Sired in 2010 to help fund expanded U.S. healthcare coverage under the Affordable Care Act (ACA), the device tax imposed a 2.3 percent levy on the medical supply sales. It applied broadly to a range of products, including pacemakers, artificial joints, surgical gloves, and dental instruments.
The levy raised $913 million in the first half of 2013, or roughly 75 percent of the expected amount. Regulators expected as many as 15,000 tax-associated filings, but only 5,107 medical device tax forms were actually filed, government statistics show.
One of the main impacts of the tax, according to industry leaders, was its decimation of the funding necessary to drive medtech innovation and fuel growth. For many manufacturers, the levy significantly hampered their ability to invest in new time- and cost-saving technologies like automation software, artificial intelligence, and robotics.
The tax also led to countless hiring freezes and in some cases, considerable layoffs. “As well as lacking effectiveness, the tax has many costly consequences for manufacturers, and was particularly crippling to smaller companies, which were forced to face challenges such as layoffs, cuts to research and development efforts, and delayed expansion plans,” Jennifer Ryan, a GlobalData analyst covering medical devices, said when Obama suspended the levy.
“The tax also threatened to seize much of the money spent on product innovation and advancement in the U.S. medical device market, which was already struggling under stringent regulatory and reimbursement procedures.”
Without the added struggle of the device tax, medtech firms can now afford to replenish their staffs and funnel resources into research and new product development. Warsaw, Ind.-based Orthopediatrics Corp., for example, plans to invest in new projects, while noninvasive patient monitoring technology provider Masimo intends to boost both its R&D and infrastructure expenditures. Endourological treatment technology developer NxThera Inc., meanwhile, is boosting its staff of researchers and sales folk.
Such strategies are likely to become more widespread as medtech companies work the tax reprieve to their advantage. Indeed, more than two-thirds (69 percent) of corporate executives intend to use freed-up device tax funds to hire more U.S.-based employees, according to a Medical Imaging & Technology Alliance (MITA) survey conducted earlier this year. Seventy-seven percent of respondents plan to invest additional resources in R&D, 69 percent hope to finance new infrastructure, and 77 percent aim to complete one or more projects currently in development.
“These survey findings confirm that suspension of the medical device tax has already helped boost investment in R&D and ignite medical technology innovation in just a few months,” MITA Board Chairman Nelson Mendes, president/CEO of Ziehm Imaging Inc., said in a July news release. “Full repeal of this burdensome tax will turn yesterday’s economic headwinds into tomorrow’s tailwinds, spurring sustained growth and protecting patient care. We appreciate the bipartisan efforts of Congress to address this tax and urge them to vote for full repeal when the time comes.”
If the time comes, that is.
The tax’s future is unclear at the moment, since a new presidential administration and potentially new U.S. Congress will ultimately determine its fate. Still, industry leaders are hopeful the suspension will eventually become permanent.
“We are optimistic,” said Clayton Hall, vice president of government affairs for the Washington, D.C.-based Medical Device Manufacturers Association (MDMA). “It’s easier to keep a tax suspended than to get it turned off.”
“I think it will be very difficult to turn it back on,” agreed Perry De Fazio, a vice president at Boston, Mass.-based investment banking firm Covington Associates. “If there is an attempt to turn [the tax] back on, there will be the question of what are you turning it back on for?”
To reconcile the shortfall in ACA funding, perhaps.
“While some camps will be legitimately relieved at the repeal of these levies, the simple reality is that the funds from both the Cadillac tax and the medical device tax are important to funding the Affordable Care Act,” William Bithoney, M.D., managing director and chief physician executive at the BDO Center for Healthcare Excellence & Innovation, noted in a statement. “How will the projected billions of dollars raised by these taxes be replaced?”
Good question.
Read more: http://bit.ly/yir1606
Mylan’s EpiPen Maelstrom
Never underestimate the wrath of an angry parent.
Mylan N.V. learned that lesson well this past summer after the pharmaceutical giant’s exorbitant price hike for the EpiPen came to light, just in time for the new school year.
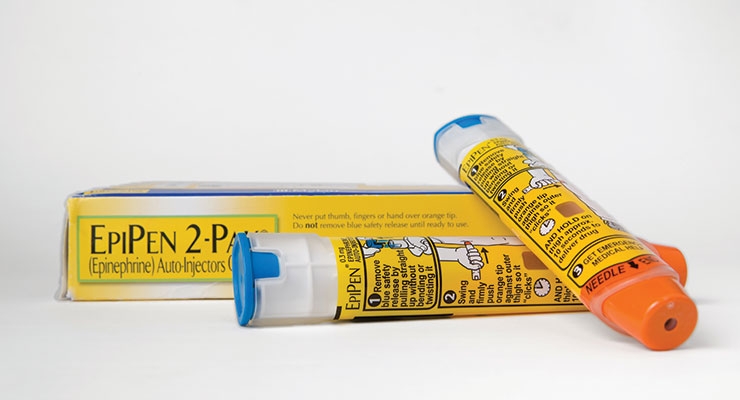
EpiPen’s exorbitant price hike angered consumers and
triggered a Congressional showdown in September with
Mylan’s CEO.
The company is a relatively newer player to the epinephrine autoinjection market, having only acquired the EpiPen brand from Merck & Co. Inc. in 2007—40 years after the product’s drug delivery system was first patented. The EpiPen’s cost upon the deal’s closing was roughly $100; now it’s up to $609, with most of the 509 percent spike occurring in the last three years, according to The New York Times.
As word spread about the exorbitant price increase, so did public outrage: An online petition protesting the accretion garnered 48,000 signatures and more than 95,600 comments (“Big Pharma at its worst,” wrote Seth E. from Castleton-On-Hudson, N.Y.). Consumer rights advocates, meanwhile, pressed for a price reduction, and members of Congress demanded an explanation for the hike.
Mylan CEO Heather Bresch blamed the rising cost of EpiPens on the insurance industry and the spread of “high-deductible” insurance plans that require patients to pay high out-of-pocket costs. “Everybody should be frustrated,” Bresch told CNBC in late August. “The patient is paying twice. They’re paying full retail price at the counter, and they’re paying higher premiums on their insurance. It was never intended that a consumer, that the patients would be paying list price, never. The system wasn’t built for that. I am hoping that this is an inflection point for this country. Our health care is in crisis. It’s no different than the mortgage financial crisis back in 2007.”
Bresch’s comments, however, only infuriated lawmakers and hastened her ascension to the corporate greed throne.
“You virtually have a monopoly, and used it to your advantage, but unfortunately, it is at the expense of the people who need it,” U.S. Rep. Gerald Connolly (D-Va.) fumed during a brutal late-September hearing.
“You never anticipated [an outcry]?” U.S. Rep. Jason Chaffetz (R-Utah) asked incredulously. “You raised the price, what did you think was going to happen?”
Probably the same thing that happened with most of the 16 other price increases: nothing. After permitting competition in the market (through a 2012 legal agreement), Mylan stepped up the pace of its price hikes. Few eyebrows were raised.
Last year, the company gained a virtual monopoly on EpiPens (as Connolly duly noted) after a voluntary recall felled its only competitor, Sanofi’s Auvi-Q, over possible dosage miscalculations.
Still more radio silence.
In an attempt to quell the furor and polish its tarnished image, Mylan expanded access and increased benefits to programs it uses to help consumers pay less for the product. It also agreed to sell a generic version of the EpiPen for about half the cost of the branded device. But the company never lowered EpiPen’s price.
And why would it? EpiPen is Mylan’s top-selling product, generating more than $1 billion of the company’s $9.5 billion in total sales last year. More than 3.6 million EpiPen prescriptions were written in 2015, according to IMS Health, with nearly 70 percent of those scripts meant for commercially insured patients.
The company’s goodwill efforts did little to quell public anger over EpiPen’s price increase, since ultimately, the brand-name product cost remained unchanged. Exacerbating the furor was Mylan’s alleged exploitation of the U.S. government through a misclassification of its epinephrine autoinjector for Medicaid rebate purposes.
The Centers for Medicare and Medicaid Services and three U.S. senators accused Mylan of overcharging taxpayers and Uncle Sam by millions of dollars by classifying its autoinjector as a generic drug, which implies that it has multiple competitors, rather than as a branded product with limited to no competition.
As a generic, a company must pay a 13 percent Medicaid rebate back to the federal government; for brand-name products, that rebate jumps to 23.1 percent. Mylan, queerly, had classified the EpiPen as a generic.
To fix its mistake, the company agreed to pay the government $465 million, though it stopped short of admitting any wrongdoing in the matter. In addition, Mylan lowered its earnings guidance to between $4.70 and $4.90 per share, compared to a previous estimate of $4.85-$5.15, citing the financial implications of the changes to its patient access programs for the EpiPen, an injector containing a drug to treat severe, life-threatening allergic reactions.
Leerink Partners analyst Jason Gerberry believes the settlement and subsequent guidance cut is a small price to pay for ending the controversy. “EpiPen can now fade off into the sunset, removing a major headache for management,” he told Bloomberg. “The settlement likely gets the U.S. government out of Mylan’s hair.”
Other experts disagree, noting that the company has received an inquiry from the U.S. Securities and Exchange Commission, and the House Oversight Committee is collecting internal corporate documents.
“Investors will like the settlement, as many will think this puts the issue largely behind the company—we disagree,” Wells Fargo analyst David Maris countered. “It does nothing to answer the original issue—EpiPen pricing and consumers.”
Read more: http://bit.ly/yir1603
Abbott and Alere’s Rocky Courtship
It seemed like a good match.
Indeed, when Abbott Laboratories and global diagnostics provider Alere Inc. announced their intended betrothal in February, both companies sounded like giddy teenage suitors. Abbott Chairman/CEO Miles D. White boasted that the union would create the “world’s premiere point-of-care testing business,” while Alere’s top executive called the partnership an “exciting and transformative milestone.”
Surely, the couple was destined for happiness. Or so it appeared.
The relationship, however, quickly soured as the pair worked through the details of its $5.8 billion merger. The first complication was Alere’s inability to file its 2015 annual report on time due to “revenue recognition in Africa and China.” The company eventually filed in early August, reporting a 3.63 percent decrease in net product sales last year.
The duo’s bond was further tested this past spring by federal investigations into Alere’s sales and practices in Africa, Asia, and Latin America, as well as potential bribery concerns. At that point, Abbott looked for a way out of the relationship—it offered to pay Alere between $30 million and $50 million to end the planned merger, but Alere rejected the overture.
The pair drifted further apart over the summer after squabbling over Alere’s extended filing delay of its annual report. “Alere has...failed to provide an adequate explanation for the extended filing delay and has refused to provide detailed and relevant information on several outstanding issues,” Abbott spokesman Scott Stoffel told Bloomberg on Aug. 8.
Alere’s retort was short and pointed: “While Abbott is free to express concerns about anything it wants, it should not imply that Abbott has any basis to avoid closing the merger...”
Relations between the two turned even frostier after Alere sued Abbott on Aug. 26 in federal court to force the larger company to fulfill its M&A obligations. Alere’s lawsuit accuses Abbott of suffering from “buyer’s remorse,” contending its executives vowed to make life difficult for the smaller company if it refused to end the deal. Specifically, the complaint claims Abbott sought interviews with roughly 33 Alere employees worldwide and demanded that Alere produce 1 million pages of documents. In addition, Alere argues that Abbott is dragging its feet on antitrust-related requests.
Abbott, though, blames Alere for the delay in securing regulatory approval (by April 2017, according to the merger agreement), citing the company’s inability to file its 2015 annual report on time. Abbott spokeswoman Darcy Ross said the healthcare behemoth is “not in any way refusing to perform any of its obligations under the antitrust provisions of the merger agreement.” She called Alere’s complaint “nonsense and without merit.”
The pair sought professional help in settling their differences, but a court-appointed mediator failed earlier this fall to resolve the case.
With the dispute still pending, both sides are potentially making future deals more complicated for themselves, industry analysts claim. Abbott’s prospective M&A candidates, for instance, could now insist on tighter parameters (like higher termination fees) to reduce risk. “I do think that some of the potential targets will probably want to think very carefully about how any potential deals are structured,” Morningstar senior analyst Debbie Wang told TheStreet in September.
The Abbott-Alere dispute might also prompt companies to more carefully consider the kind of targets they pursue and their potential value. Wang noted that investors have long been wary about Abbott’s deal for Alere, “even before all the skeletons began falling out of the closet.”
Read more: http://bit.ly/yir1602
Pulling the Plug on Patient Preference
It was a slick move on UnitedHealthcare’s part: Announce an important and potentially contentious policy change but disclose it in the most furtive manner possible—explain it, perhaps, on a random page well within the body of a lengthy member bulletin and then wait for people to notice (or not).
The strategy would have been successful, too, had it not been for the sharp eye of a rival company executive who graciously helped publicize the policy change in early May and jumpstart its ensuing furor.
It didn’t take much to ignite the firestorm, actually—UnitedHealthcare’s new protocol virtually guaranteed a public pushback, as it designated Medtronic plc as the insurer’s preferred insulin pump supplier beginning July 1.
UnitedHealthcare (UHC) executives tried putting a positive spin on the policy change by tying the exclusive agreement to “ongoing efforts to provide a better member experience, while increasing quality” and lowering the overall cost of U.S. diabetes care. Diabetics, however, weren’t buying it. They dismissed the marketing jargon and lambasted the insurance carrier for limiting patient choice as well as stifling innovation.
“Patients are concerned about single-source contracts that limit choice to one therapeutic option,” the diaTribe Foundation wrote in an advocacy letter sent to payers in the first week of May. “These therapies and technologies are complex, and shifting patients to one specific product can result in weaker diabetes management. Open competition between diabetes companies may prompt faster innovation and lower prices.”
“I appreciate the ability to choose. I can’t un-choose diabetes, but deciding what tools I use to manage this disease is a choice I value highly,” diabetes blogger Kerri Sparling ranted in a May 4 posting. “Since I started pumping insulin in 2004, I’ve used Medtronic, Animas, and Tandem insulin pumps, and have had good experiences with them all. I like them, as devices, for different reasons, and being able to switch to the pump that suits my needs best in different stages in my life has been important in taking care of me as a whole patient. We’re not talking about T-shirts here; this is a medical device that is part of every moment of every day, and you’d better believe that access to having a choice matters.”
Ironically, UHC claims to have based its new policy on member choice. A digital fact sheet explaining the UHC-Medtronic relationship shows an overwhelming majority of UnitedHealthcare patients favoring Medtronic insulin pumps over all other brands. In fact, less than 2,500 requests are submitted annually for other manufacturers’ devices (excluding children 18 and younger and Medicare plans), UHC notes.
To its credit, UHC’s new policy includes several exemptions: children under 18 as well as Medicare Advantage, UHC Sierra Health, and Life Commercial plan members. There also is a clinical exemption process for cases in which medical needs require use of a non-Medtronic pump. Approved requests are covered as an in-network benefit.
Covered pumps under UnitedHealthcare’s new policy include Medtronic’s Mini-Med 530G, which has an integrated pump and a continuous glucose monitoring system with Threshold Suspend, a feature that temporarily shuts off insulin delivery if sensors show glucose levels falling below a preset amount.
UHC executives insist the policy change was driven by patient safety, service, and cost. Officials specifically cited the 2012 ASPIRE study, which found that pumps with a low-glucose threshold-suspend feature (like the one on the Mini-Med 530G) can help reduce the frequency and duration of hypoglycemic events.
Such data is of little comfort, though, to medtech experts and patients rights advocates who remain troubled by the repercussions of exclusionary contracts. “We see the Medtronic-UnitedHealthcare deal as tremendously worrisome,” MDMA’s Hall said. “This is an exclusionary contract. You can be on a pump and it it’s working fine—you think it’s the best pump for you and your clinician thinks it’s the best pump for you. But if that [pump’s] warranty expires today, UHC and Medtronic are saying, ‘No, you can’t have the pump that works best for you.’ The diabetes community is very upset with the device maker in this case because the device maker has been an important partner in working toward solutions. Maybe the market will figure this out, but we think this deal is very worrisome.”
Read more: http://bit.ly/yir1604
More Merger Mania
The healthcare analysts at J.P. Morgan Chase & Co. really nailed it this year.
Their predictions for medtech M&A activity were remarkably accurate (for the most part). In fact, it almost seemed as if the group was peering into a crystal ball when describing the 2016 landscape.
Granted, the team’s overall conclusions weren’t all that difficult to reach, given the record sum spent on mergers and acquisitions last year ($127 billion in the fewest number of deals since 2009). More impressive, however, is the forecast’s mastery of details—buyers’ names, potential acquisition totals, and deal sizes.
Consider the following prediction, extracted from a report released at J.P. Morgan’s Healthcare conference in early January: “We see Abbott as likely the most aggressive acquirer, pursuing potential acquisitions in three of its four verticals—namely branded generics, diagnostics, and medical devices [specifically vascular]...and, given the level of potential activity, [we] wouldn’t be surprised to hear something early in the year.”
As if on cue, Abbott embarked on its shopping spree less than a month later, purchasing global point-of-care testing firm Alere Inc. for $5.8 billion on Feb. 1. The deal is expected to strengthen Abbott’s diagnostic offerings, creating the “world’s premiere point-of-care testing business,” according to Abbott Chairman/CEO Miles D. White.
The acquisition is awaiting regulatory approval, though both companies are caught up in a welter of lawsuits and federal probes that could take months or more to resolve. In public, both Abbott and Alere insist they remain committed to the deal, but court documents reveal mutual rancor, mistrust, and uncertainty as to whether the merger will close. Too bad J.P. Morgan analysts couldn’t have foreseen the pair’s troubles.
They were correct, however, about Abbott’s next target, St. Paul, Minn.-based St. Jude Medical Inc. The $25 billion deal—announced April 28 (undoubtedly blindsiding Alere executives)—diversifies its heart stent business and enables the company to compete more effectively against larger rivals Medtronic plc and Boston Scientific Corp.
“In an industry where a larger portfolio is increasingly important, Abbott just built a real medtech franchise,” Joanne Wuensch, an analyst at BMO Capital Markets Corp., said in a note to investors upon learning of the proposed acquisition. “From our perspective, there is very little, if any, product overlap, but a real opportunity for cross-selling.”
To satisfy anti-trust regulators, both Abbott and St. Jude sold portions of their businesses to Terumo Corporation for $1.12 billion in mid-October. The sale involved St. Jude’s Angio-Seal and Femoseal vascular closure products and Abbott’s Vado Steerable Sheath.
Abbott’s turn from buyer to seller actually commenced a month before the Terumo transaction with the $4.33 billion sale of its Medical Optics business to Johnson & Johnson. The deal is expected to help J&J expand its eye care portfolio with products in cataract surgery, laser refractive surgery, and consumer eye health.
Although it was not specifically presaged from Abbott’s perspective, J&J’s eye care purchase actually fulfills another prediction by J.P. Morgan analysts: Johnson & Johnson’s return to the M&A market.
Starting the year with $37 billion in cash and just $20 billion in debt, J&J was ideally suited to take advantage of strategic deals. And it did so quite sensibly, acquiring NeuWave Medical (through its Ethicon unit) and privately held BioMedical Enterprises (through its DePuy Synthes division). The NeuWave Medical deal broadens Ethicon’s surgical offerings, particularly in surgical oncology, while the BioMedical purchase strengthens DePuy’s position in elective extremity surgery, the fastest-growing segment in orthopedics.
The latter two purchases corroborate J.P. Morgan’s forecast for “smaller deals” from J&J and other “likely acquirers” such as Stryker Corp., which bought Synergetics USA Inc.’s neuro portfolio, the CareFusion vertebral compression fracture portfolio from BD, and Stanmore Implants Worldwide Limited (for 35.6 million lire) all within a month.
Other smaller deals were orchestrated by Medtronic (Bellco, as well as Smith and Nephew plc’s gynecology business), Dentsply Sirona (MIS Implants Technologies Ltd., $375 million), GE Healthcare (Biosafe Group), and Boston Scientific (Cosman Medical Inc., and EndoChoice Holdings Inc., $210 million).
There were, of course, larger deals as well, although none matched the scope of the $43 billion Medtronic-Covidien merger last year. The Abbott-St. Jude union topped 2016’s list (through late October, anyway); runners-up included J&J’s deal for Abbott’s eye care business, Thermo Fisher Scientific Inc.’s $4.2 billion purchase of FEI Company and its $1.3 billion bid for Affymetrix Inc., Stryker’s $2.77 billion purchase of Sage Products LLC and its $1.28 billion purchase of Physio-Control International Inc., Medtronic’s $1.1 billion purchase of Heartware International Inc., and Zimmer Biomet’s $1 billion purchase of LDR Holding Corp.
“[2015] was a record year in terms of [M&A] activity for the healthcare sector,” J.P. Morgan analysts wrote in their report. “...We expect the tone to continue—maybe [it won’t be] as robust, but definitely very active in the next coming year.”
Active, indeed.
Read more: http://bit.ly/yir1601
In many respects, 2016 has seemed like a continuation of its predecessor. The lines between medtech, health IT, healthcare services, and even therapeutics blurred more this year with Medtronic plc’s investments in consulting and hospital-managed services, and Johnson & Johnson’s participation in the setup of Verb Surgical, its robotic surgery joint venture with Verily, née Google Life Sciences.
The drive for scale and strength among OEMs continued with Abbott Laboratories’ $25 billion bid for St. Jude Medical Inc., Thermo Fisher Scientific Inc.’s $4.2 billion purchase of FEI Company, Stryker Corp.’s $2.77 billion acquisition of Sage Products LLC, and Medtronic’s $1.1 billion purchase of Heartware International Inc. Likewise, companies also maintained their affinity in 2016 for bolt-on transactions to fill portfolio gaps and divestitures to exit non-core competency areas or shed poorly-performing business units.
Big data and cybersecurity issues lingered well into the year too, as the industry began digesting guidance from the U.S. Food and Drug Administration (FDA) on the use of real-world data, as well as recommendations on mitigating device cybersecurity threats following the 2015 discovery of defensive vulnerabilities in Hospira’s infusion pump systems.
The year, however, was not without its own defining moments, though. The most notable, obviously, was the suspension of the 2.3 percent medical device tax—a longstanding source of contention between industry leaders and Obamacare proponents. There was also the reclassification of transvaginal surgical mesh (from Class II to Class III), the FDA’s second-ever device ban (on powdered surgical gloves), the release of a redesigned (and presumably safer) duodenoscope, and the official coronation of a new FDA commissioner.
For insight into these and other kairos of the past 10 months, take a stroll down memory lane, courtesy of Medical Product Outsourcing.
Theranos’ Terrible, Horrible, No Good Very Bad Year
It wasn’t supposed to turn out this way.

In June, Forbes cut Theranos CEO Elizabeth Holmes’ net worth
from an estimated $4.5 billion to 0.
Holmes’ quest to do good turned inexorably bad relatively quickly, though. As her company, Theranos Inc., steadily gained in valuation—eventually reaching $9 billion—clinical pathologists and biochemists became skeptical of the technology the firm developed to measure up to 70 biomarkers from a single finger-prick blood sample. Some experts criticized the lack of peer review for the tests; others lambasted the secrecy surrounding the testing. Exacerbating those concerns was an August 2015 FDA inspection that found uncleared medical devices and objectionable conditions inside Theranos’ facilities.
The company (and Holmes) suffered a more damaging blow the following month after a Wall Street Journal investigation accused Theranos of using its flagship technology for just a small number of tests, relying on conventional devices for most assays, and releasing questionable results to patients.
Now, Theranos’ fairy tale-like existence seems destined for an unhappily-ever-after ending. Since the Journal article appeared last fall, the company has voided tens of thousands of test results, faces federal civil and criminal investigations, and is appealing the revocation of its blood testing license at its Newark, Calif., lab. This year has been particularly challenging for the firm: In January, the Centers for Medicare and Medicaid Services released a scathing inspection report, prompting Walgreens to terminate its three-year partnership with the company and shutter all Theranos lab-testing services at its retail locations.
In June, Forbes cut Holmes’ net worth to zero, based on the assumption that Theranos was worth roughly $800 million—the estimated value of its investment capital. Then in October, the embattled company laid off 340 people (about 43 percent of its staff) as it pulled the plug on its clinical labs and Theranos Wellness centers in California, Arizona, and Texas.
Holmes’ dream was officially dead.
Rather than mourn, however, Holmes set her sights on a new goal: Developing the miniLab, a 95-pound, tabletop-sized diagnostic tool designed to reduce the cost and improve the accessibility of blood tests. “Our ultimate goal is to commercialize miniaturized, automated laboratories capable of small-volume sample testing, with an emphasis on vulnerable patient populations, including oncology, pediatrics, and intensive care,” Holmes wrote in an Oct. 5 blog post on the company’s website. “We have a new executive team leading our work toward obtaining FDA clearances, building commercial partnerships, and pursuing publications in scientific journals. We are fortunate to have supporters and investors who believe deeply in our mission of affordable, less invasive lab testing, and to have the runway to realize our vision.”
Theranos’ new vision is not quite ready for takeoff, though. Less than a week after announcing the pivot plan, a major hedge fund investor sued the company and Holmes for “knowingly and repeatedly” lying to attract $96 million in financing. An Oct. 10 letter to investors from San Francisco, Calif.-based Partner Fund Management LP accused Theranos and its CEO/founder of engaging in securities fraud and other violations by inducing the hedge fund to invest in the startup, according to the Journal and Forbes.
“Among other things, Theranos and its principals knowingly and repeatedly lied that they had developed proprietary technologies that worked, were on the cusp of receiving all necessary regulatory clearances, and approvals, and concealed the truth about the commercial viability of their technologies and methods,” Forbes reported, quoting the letter.
Theranos, in turn, denied the charges, and countered with its own claims: “The suit is without merit, the assertions are baseless, and the plaintiff is engaging in revisionist history,” the company said in an official statement. “Most of the company statements the plaintiff has cited in its suit were made after the time the plaintiff invested, and could not possibly have been the original basis for investment. This wholesale reliance on post-investment statements, therefore, negates the claim that the plaintiff was misled.”
PFM’s lawsuit, filed under seal in Delaware Chancery Court, seeks to recoup the firm’s investment plus damages and costs associated with the claim, the Journal noted.
Although it remains emphatic about its innocence, PFM’s allegations create a headache for Theranos as it attempts to start anew. The lawsuit could potentially prompt other financiers to seek retribution for lost investments, or worse, draw further scrutiny from federal investigators. At the very least, it will likely further sully the company’s (and Holmes’) already tarnished reputation, possibly leading to future problems with investors and government regulators. Such setbacks could eventually threaten Theranos’ existence.
As Forbes’ Matthew Herper opined when Theranos announced its pivot: “The wax on this company’s wings has melted, and it is not clear when Theranos will hit the ground.”
Prepare for impact.
Read more: http://bit.ly/yir1605
The Tax Man Leaveth
The day went largely unnoticed. There were no formal celebrations marking the moment, no proclamations immortalizing the date, and no special gatherings acknowledging the occasion. The day, in fact, was no different than any other that week. Except that it was a Monday. The start of a new workweek. The beginning of a new month.
Monday, Feb. 1 was also the start of a new era for the medtech industry: It was the final medical device excise tax payment deadline.
For two years, anyway. Or perhaps, ever.
Long a thorn in the industry’s side, the 2.3 percent medical device tax was suspended late last year through 2017 as part of a compromise $1.8 trillion spending package that prevented a government shutdown. The suspension capped a long, hard-fought repeal effort by MDMA, the Advanced Medical Technology Association, and other lobbying groups that opposed the levy for its chilling effect on innovation and R&D, as well as its drain on corporate finances. The fight triggered at least five showdowns in the U.S. House of Representatives, two of which featured stand-alone repeal measures, and two involved inclusions in a jobs package and a reconciliation vote.
Sired in 2010 to help fund expanded U.S. healthcare coverage under the Affordable Care Act (ACA), the device tax imposed a 2.3 percent levy on the medical supply sales. It applied broadly to a range of products, including pacemakers, artificial joints, surgical gloves, and dental instruments.
The levy raised $913 million in the first half of 2013, or roughly 75 percent of the expected amount. Regulators expected as many as 15,000 tax-associated filings, but only 5,107 medical device tax forms were actually filed, government statistics show.
One of the main impacts of the tax, according to industry leaders, was its decimation of the funding necessary to drive medtech innovation and fuel growth. For many manufacturers, the levy significantly hampered their ability to invest in new time- and cost-saving technologies like automation software, artificial intelligence, and robotics.
The tax also led to countless hiring freezes and in some cases, considerable layoffs. “As well as lacking effectiveness, the tax has many costly consequences for manufacturers, and was particularly crippling to smaller companies, which were forced to face challenges such as layoffs, cuts to research and development efforts, and delayed expansion plans,” Jennifer Ryan, a GlobalData analyst covering medical devices, said when Obama suspended the levy.
“The tax also threatened to seize much of the money spent on product innovation and advancement in the U.S. medical device market, which was already struggling under stringent regulatory and reimbursement procedures.”
Without the added struggle of the device tax, medtech firms can now afford to replenish their staffs and funnel resources into research and new product development. Warsaw, Ind.-based Orthopediatrics Corp., for example, plans to invest in new projects, while noninvasive patient monitoring technology provider Masimo intends to boost both its R&D and infrastructure expenditures. Endourological treatment technology developer NxThera Inc., meanwhile, is boosting its staff of researchers and sales folk.
Such strategies are likely to become more widespread as medtech companies work the tax reprieve to their advantage. Indeed, more than two-thirds (69 percent) of corporate executives intend to use freed-up device tax funds to hire more U.S.-based employees, according to a Medical Imaging & Technology Alliance (MITA) survey conducted earlier this year. Seventy-seven percent of respondents plan to invest additional resources in R&D, 69 percent hope to finance new infrastructure, and 77 percent aim to complete one or more projects currently in development.
“These survey findings confirm that suspension of the medical device tax has already helped boost investment in R&D and ignite medical technology innovation in just a few months,” MITA Board Chairman Nelson Mendes, president/CEO of Ziehm Imaging Inc., said in a July news release. “Full repeal of this burdensome tax will turn yesterday’s economic headwinds into tomorrow’s tailwinds, spurring sustained growth and protecting patient care. We appreciate the bipartisan efforts of Congress to address this tax and urge them to vote for full repeal when the time comes.”
If the time comes, that is.
The tax’s future is unclear at the moment, since a new presidential administration and potentially new U.S. Congress will ultimately determine its fate. Still, industry leaders are hopeful the suspension will eventually become permanent.
“We are optimistic,” said Clayton Hall, vice president of government affairs for the Washington, D.C.-based Medical Device Manufacturers Association (MDMA). “It’s easier to keep a tax suspended than to get it turned off.”
“I think it will be very difficult to turn it back on,” agreed Perry De Fazio, a vice president at Boston, Mass.-based investment banking firm Covington Associates. “If there is an attempt to turn [the tax] back on, there will be the question of what are you turning it back on for?”
To reconcile the shortfall in ACA funding, perhaps.
“While some camps will be legitimately relieved at the repeal of these levies, the simple reality is that the funds from both the Cadillac tax and the medical device tax are important to funding the Affordable Care Act,” William Bithoney, M.D., managing director and chief physician executive at the BDO Center for Healthcare Excellence & Innovation, noted in a statement. “How will the projected billions of dollars raised by these taxes be replaced?”
Good question.
Read more: http://bit.ly/yir1606
Mylan’s EpiPen Maelstrom
Never underestimate the wrath of an angry parent.
Mylan N.V. learned that lesson well this past summer after the pharmaceutical giant’s exorbitant price hike for the EpiPen came to light, just in time for the new school year.

EpiPen’s exorbitant price hike angered consumers and
triggered a Congressional showdown in September with
Mylan’s CEO.
As word spread about the exorbitant price increase, so did public outrage: An online petition protesting the accretion garnered 48,000 signatures and more than 95,600 comments (“Big Pharma at its worst,” wrote Seth E. from Castleton-On-Hudson, N.Y.). Consumer rights advocates, meanwhile, pressed for a price reduction, and members of Congress demanded an explanation for the hike.
Mylan CEO Heather Bresch blamed the rising cost of EpiPens on the insurance industry and the spread of “high-deductible” insurance plans that require patients to pay high out-of-pocket costs. “Everybody should be frustrated,” Bresch told CNBC in late August. “The patient is paying twice. They’re paying full retail price at the counter, and they’re paying higher premiums on their insurance. It was never intended that a consumer, that the patients would be paying list price, never. The system wasn’t built for that. I am hoping that this is an inflection point for this country. Our health care is in crisis. It’s no different than the mortgage financial crisis back in 2007.”
Bresch’s comments, however, only infuriated lawmakers and hastened her ascension to the corporate greed throne.
“You virtually have a monopoly, and used it to your advantage, but unfortunately, it is at the expense of the people who need it,” U.S. Rep. Gerald Connolly (D-Va.) fumed during a brutal late-September hearing.
“You never anticipated [an outcry]?” U.S. Rep. Jason Chaffetz (R-Utah) asked incredulously. “You raised the price, what did you think was going to happen?”
Probably the same thing that happened with most of the 16 other price increases: nothing. After permitting competition in the market (through a 2012 legal agreement), Mylan stepped up the pace of its price hikes. Few eyebrows were raised.
Last year, the company gained a virtual monopoly on EpiPens (as Connolly duly noted) after a voluntary recall felled its only competitor, Sanofi’s Auvi-Q, over possible dosage miscalculations.
Still more radio silence.
In an attempt to quell the furor and polish its tarnished image, Mylan expanded access and increased benefits to programs it uses to help consumers pay less for the product. It also agreed to sell a generic version of the EpiPen for about half the cost of the branded device. But the company never lowered EpiPen’s price.
And why would it? EpiPen is Mylan’s top-selling product, generating more than $1 billion of the company’s $9.5 billion in total sales last year. More than 3.6 million EpiPen prescriptions were written in 2015, according to IMS Health, with nearly 70 percent of those scripts meant for commercially insured patients.
The company’s goodwill efforts did little to quell public anger over EpiPen’s price increase, since ultimately, the brand-name product cost remained unchanged. Exacerbating the furor was Mylan’s alleged exploitation of the U.S. government through a misclassification of its epinephrine autoinjector for Medicaid rebate purposes.
The Centers for Medicare and Medicaid Services and three U.S. senators accused Mylan of overcharging taxpayers and Uncle Sam by millions of dollars by classifying its autoinjector as a generic drug, which implies that it has multiple competitors, rather than as a branded product with limited to no competition.
As a generic, a company must pay a 13 percent Medicaid rebate back to the federal government; for brand-name products, that rebate jumps to 23.1 percent. Mylan, queerly, had classified the EpiPen as a generic.
To fix its mistake, the company agreed to pay the government $465 million, though it stopped short of admitting any wrongdoing in the matter. In addition, Mylan lowered its earnings guidance to between $4.70 and $4.90 per share, compared to a previous estimate of $4.85-$5.15, citing the financial implications of the changes to its patient access programs for the EpiPen, an injector containing a drug to treat severe, life-threatening allergic reactions.
Leerink Partners analyst Jason Gerberry believes the settlement and subsequent guidance cut is a small price to pay for ending the controversy. “EpiPen can now fade off into the sunset, removing a major headache for management,” he told Bloomberg. “The settlement likely gets the U.S. government out of Mylan’s hair.”
Other experts disagree, noting that the company has received an inquiry from the U.S. Securities and Exchange Commission, and the House Oversight Committee is collecting internal corporate documents.
“Investors will like the settlement, as many will think this puts the issue largely behind the company—we disagree,” Wells Fargo analyst David Maris countered. “It does nothing to answer the original issue—EpiPen pricing and consumers.”
Read more: http://bit.ly/yir1603
Abbott and Alere’s Rocky Courtship
It seemed like a good match.
Indeed, when Abbott Laboratories and global diagnostics provider Alere Inc. announced their intended betrothal in February, both companies sounded like giddy teenage suitors. Abbott Chairman/CEO Miles D. White boasted that the union would create the “world’s premiere point-of-care testing business,” while Alere’s top executive called the partnership an “exciting and transformative milestone.”
Surely, the couple was destined for happiness. Or so it appeared.
The relationship, however, quickly soured as the pair worked through the details of its $5.8 billion merger. The first complication was Alere’s inability to file its 2015 annual report on time due to “revenue recognition in Africa and China.” The company eventually filed in early August, reporting a 3.63 percent decrease in net product sales last year.
The duo’s bond was further tested this past spring by federal investigations into Alere’s sales and practices in Africa, Asia, and Latin America, as well as potential bribery concerns. At that point, Abbott looked for a way out of the relationship—it offered to pay Alere between $30 million and $50 million to end the planned merger, but Alere rejected the overture.
The pair drifted further apart over the summer after squabbling over Alere’s extended filing delay of its annual report. “Alere has...failed to provide an adequate explanation for the extended filing delay and has refused to provide detailed and relevant information on several outstanding issues,” Abbott spokesman Scott Stoffel told Bloomberg on Aug. 8.
Alere’s retort was short and pointed: “While Abbott is free to express concerns about anything it wants, it should not imply that Abbott has any basis to avoid closing the merger...”
Relations between the two turned even frostier after Alere sued Abbott on Aug. 26 in federal court to force the larger company to fulfill its M&A obligations. Alere’s lawsuit accuses Abbott of suffering from “buyer’s remorse,” contending its executives vowed to make life difficult for the smaller company if it refused to end the deal. Specifically, the complaint claims Abbott sought interviews with roughly 33 Alere employees worldwide and demanded that Alere produce 1 million pages of documents. In addition, Alere argues that Abbott is dragging its feet on antitrust-related requests.
Abbott, though, blames Alere for the delay in securing regulatory approval (by April 2017, according to the merger agreement), citing the company’s inability to file its 2015 annual report on time. Abbott spokeswoman Darcy Ross said the healthcare behemoth is “not in any way refusing to perform any of its obligations under the antitrust provisions of the merger agreement.” She called Alere’s complaint “nonsense and without merit.”
The pair sought professional help in settling their differences, but a court-appointed mediator failed earlier this fall to resolve the case.
With the dispute still pending, both sides are potentially making future deals more complicated for themselves, industry analysts claim. Abbott’s prospective M&A candidates, for instance, could now insist on tighter parameters (like higher termination fees) to reduce risk. “I do think that some of the potential targets will probably want to think very carefully about how any potential deals are structured,” Morningstar senior analyst Debbie Wang told TheStreet in September.
The Abbott-Alere dispute might also prompt companies to more carefully consider the kind of targets they pursue and their potential value. Wang noted that investors have long been wary about Abbott’s deal for Alere, “even before all the skeletons began falling out of the closet.”
Read more: http://bit.ly/yir1602
Pulling the Plug on Patient Preference
It was a slick move on UnitedHealthcare’s part: Announce an important and potentially contentious policy change but disclose it in the most furtive manner possible—explain it, perhaps, on a random page well within the body of a lengthy member bulletin and then wait for people to notice (or not).
The strategy would have been successful, too, had it not been for the sharp eye of a rival company executive who graciously helped publicize the policy change in early May and jumpstart its ensuing furor.
It didn’t take much to ignite the firestorm, actually—UnitedHealthcare’s new protocol virtually guaranteed a public pushback, as it designated Medtronic plc as the insurer’s preferred insulin pump supplier beginning July 1.
UnitedHealthcare (UHC) executives tried putting a positive spin on the policy change by tying the exclusive agreement to “ongoing efforts to provide a better member experience, while increasing quality” and lowering the overall cost of U.S. diabetes care. Diabetics, however, weren’t buying it. They dismissed the marketing jargon and lambasted the insurance carrier for limiting patient choice as well as stifling innovation.
“Patients are concerned about single-source contracts that limit choice to one therapeutic option,” the diaTribe Foundation wrote in an advocacy letter sent to payers in the first week of May. “These therapies and technologies are complex, and shifting patients to one specific product can result in weaker diabetes management. Open competition between diabetes companies may prompt faster innovation and lower prices.”
“I appreciate the ability to choose. I can’t un-choose diabetes, but deciding what tools I use to manage this disease is a choice I value highly,” diabetes blogger Kerri Sparling ranted in a May 4 posting. “Since I started pumping insulin in 2004, I’ve used Medtronic, Animas, and Tandem insulin pumps, and have had good experiences with them all. I like them, as devices, for different reasons, and being able to switch to the pump that suits my needs best in different stages in my life has been important in taking care of me as a whole patient. We’re not talking about T-shirts here; this is a medical device that is part of every moment of every day, and you’d better believe that access to having a choice matters.”
Ironically, UHC claims to have based its new policy on member choice. A digital fact sheet explaining the UHC-Medtronic relationship shows an overwhelming majority of UnitedHealthcare patients favoring Medtronic insulin pumps over all other brands. In fact, less than 2,500 requests are submitted annually for other manufacturers’ devices (excluding children 18 and younger and Medicare plans), UHC notes.
To its credit, UHC’s new policy includes several exemptions: children under 18 as well as Medicare Advantage, UHC Sierra Health, and Life Commercial plan members. There also is a clinical exemption process for cases in which medical needs require use of a non-Medtronic pump. Approved requests are covered as an in-network benefit.
Covered pumps under UnitedHealthcare’s new policy include Medtronic’s Mini-Med 530G, which has an integrated pump and a continuous glucose monitoring system with Threshold Suspend, a feature that temporarily shuts off insulin delivery if sensors show glucose levels falling below a preset amount.
UHC executives insist the policy change was driven by patient safety, service, and cost. Officials specifically cited the 2012 ASPIRE study, which found that pumps with a low-glucose threshold-suspend feature (like the one on the Mini-Med 530G) can help reduce the frequency and duration of hypoglycemic events.
Such data is of little comfort, though, to medtech experts and patients rights advocates who remain troubled by the repercussions of exclusionary contracts. “We see the Medtronic-UnitedHealthcare deal as tremendously worrisome,” MDMA’s Hall said. “This is an exclusionary contract. You can be on a pump and it it’s working fine—you think it’s the best pump for you and your clinician thinks it’s the best pump for you. But if that [pump’s] warranty expires today, UHC and Medtronic are saying, ‘No, you can’t have the pump that works best for you.’ The diabetes community is very upset with the device maker in this case because the device maker has been an important partner in working toward solutions. Maybe the market will figure this out, but we think this deal is very worrisome.”
Read more: http://bit.ly/yir1604
More Merger Mania
The healthcare analysts at J.P. Morgan Chase & Co. really nailed it this year.
Their predictions for medtech M&A activity were remarkably accurate (for the most part). In fact, it almost seemed as if the group was peering into a crystal ball when describing the 2016 landscape.
Granted, the team’s overall conclusions weren’t all that difficult to reach, given the record sum spent on mergers and acquisitions last year ($127 billion in the fewest number of deals since 2009). More impressive, however, is the forecast’s mastery of details—buyers’ names, potential acquisition totals, and deal sizes.
Consider the following prediction, extracted from a report released at J.P. Morgan’s Healthcare conference in early January: “We see Abbott as likely the most aggressive acquirer, pursuing potential acquisitions in three of its four verticals—namely branded generics, diagnostics, and medical devices [specifically vascular]...and, given the level of potential activity, [we] wouldn’t be surprised to hear something early in the year.”
As if on cue, Abbott embarked on its shopping spree less than a month later, purchasing global point-of-care testing firm Alere Inc. for $5.8 billion on Feb. 1. The deal is expected to strengthen Abbott’s diagnostic offerings, creating the “world’s premiere point-of-care testing business,” according to Abbott Chairman/CEO Miles D. White.
The acquisition is awaiting regulatory approval, though both companies are caught up in a welter of lawsuits and federal probes that could take months or more to resolve. In public, both Abbott and Alere insist they remain committed to the deal, but court documents reveal mutual rancor, mistrust, and uncertainty as to whether the merger will close. Too bad J.P. Morgan analysts couldn’t have foreseen the pair’s troubles.
They were correct, however, about Abbott’s next target, St. Paul, Minn.-based St. Jude Medical Inc. The $25 billion deal—announced April 28 (undoubtedly blindsiding Alere executives)—diversifies its heart stent business and enables the company to compete more effectively against larger rivals Medtronic plc and Boston Scientific Corp.
“In an industry where a larger portfolio is increasingly important, Abbott just built a real medtech franchise,” Joanne Wuensch, an analyst at BMO Capital Markets Corp., said in a note to investors upon learning of the proposed acquisition. “From our perspective, there is very little, if any, product overlap, but a real opportunity for cross-selling.”
To satisfy anti-trust regulators, both Abbott and St. Jude sold portions of their businesses to Terumo Corporation for $1.12 billion in mid-October. The sale involved St. Jude’s Angio-Seal and Femoseal vascular closure products and Abbott’s Vado Steerable Sheath.
Abbott’s turn from buyer to seller actually commenced a month before the Terumo transaction with the $4.33 billion sale of its Medical Optics business to Johnson & Johnson. The deal is expected to help J&J expand its eye care portfolio with products in cataract surgery, laser refractive surgery, and consumer eye health.
Although it was not specifically presaged from Abbott’s perspective, J&J’s eye care purchase actually fulfills another prediction by J.P. Morgan analysts: Johnson & Johnson’s return to the M&A market.
Starting the year with $37 billion in cash and just $20 billion in debt, J&J was ideally suited to take advantage of strategic deals. And it did so quite sensibly, acquiring NeuWave Medical (through its Ethicon unit) and privately held BioMedical Enterprises (through its DePuy Synthes division). The NeuWave Medical deal broadens Ethicon’s surgical offerings, particularly in surgical oncology, while the BioMedical purchase strengthens DePuy’s position in elective extremity surgery, the fastest-growing segment in orthopedics.
The latter two purchases corroborate J.P. Morgan’s forecast for “smaller deals” from J&J and other “likely acquirers” such as Stryker Corp., which bought Synergetics USA Inc.’s neuro portfolio, the CareFusion vertebral compression fracture portfolio from BD, and Stanmore Implants Worldwide Limited (for 35.6 million lire) all within a month.
Other smaller deals were orchestrated by Medtronic (Bellco, as well as Smith and Nephew plc’s gynecology business), Dentsply Sirona (MIS Implants Technologies Ltd., $375 million), GE Healthcare (Biosafe Group), and Boston Scientific (Cosman Medical Inc., and EndoChoice Holdings Inc., $210 million).
There were, of course, larger deals as well, although none matched the scope of the $43 billion Medtronic-Covidien merger last year. The Abbott-St. Jude union topped 2016’s list (through late October, anyway); runners-up included J&J’s deal for Abbott’s eye care business, Thermo Fisher Scientific Inc.’s $4.2 billion purchase of FEI Company and its $1.3 billion bid for Affymetrix Inc., Stryker’s $2.77 billion purchase of Sage Products LLC and its $1.28 billion purchase of Physio-Control International Inc., Medtronic’s $1.1 billion purchase of Heartware International Inc., and Zimmer Biomet’s $1 billion purchase of LDR Holding Corp.
“[2015] was a record year in terms of [M&A] activity for the healthcare sector,” J.P. Morgan analysts wrote in their report. “...We expect the tone to continue—maybe [it won’t be] as robust, but definitely very active in the next coming year.”
Active, indeed.
Read more: http://bit.ly/yir1601
|
The irony is all but laughable. After several millennia of trial-and-error practice, Homo sapiens are finally getting better at predicting the future—only they’re now being outdone by man-made machines. The human brain, despite all its wonder, is no match for computers. It simply isn’t wired to mine data and look for patterns, or think in terms of algorithms. Thus, it is surprisingly inept at one of the key skills necessary for accurate forecasting: probabilistic reasoning—i.e., finding patterns in similar past events to determine a plausible outcome. Optimism could be at fault here. Research has shown that humans tend to overestimate the chances that the future will be different (perhaps better?) than the past. For proof, look no further than the mid-20th century forecasts of flying cars, glass-domed houses, jetpack mail delivery, space hospitals, and flying fire engines. “It can be very dangerous to make predictions,” world-renowned theoretical physicist Michio Kaku, Ph.D., warned at a recent conference. “But physicists love to make predictions. And in the early days of the internet, one physicist thought the World Wide Web would be used mainly as a forum for high culture and high art—a place for ideas. Well, the internet today is about 35 percent pornography.” Never saw that one coming. Such soothsaying gaffes could be avoided, however, through better receptivity. Research spearheaded by University of Pennsylvania Professor Philip E. Tetlock found that open-minded forecasters—namely, those willing to consider multiple explanations and balance them together before making predictions—are more accurate prognosticators than those relying on a single big idea. “What distinguishes superforecasters is their ability to put aside their opinions, at least temporarily, and just focus on accuracy,” Tetlock explained in a Wharton School interview last fall. “We found that people who scored high on psychological measures of active open-mindedness and need for cognition—those people who scored high on those personality variables tended to do quite a bit better as forecasters.” Using Tetlock’s research as a guide, Medical Product Outsourcing reached out to numerous open-minded medtech experts for their reflections on the past year and their predictions for 2017. The bold bunch of superforecasters included: Mark Bonifacio, founder and president of Boston, Mass.-based Bonifacio Consulting Services LLC, a global consulting and advisory firm to medical device OEMs, contract manufacturers, private equity, and other investors in the medtech manufacturing sector. Elizabeth Cairns, medtech reporter at EP Vantage, a daily news service covering the medtech, pharmaceutical and biotech industries (its parent company is life science market intelligence firm Evaluate Ltd.). Brian Williams, a member of PwC’s Strategy and Innovation, New Entrants, and Healthcare practices. Michael Barbella: What was the most significant medtech news in 2016? Mark Bonifacio: It is always hard to single out one event or topic in any industry, let alone one as complex and dynamic as medtech. Two that quickly come to mind are pharmaceutical pricing and the M&A activity that is rapidly changing the industry landscape at the OEM and contract manufacturing level. On the M&A front—minus the Abbott/St. Jude Medical tie-up—the size of the deals may have declined a bit, but the pace is still very healthy. And there really are no short-term signs that this [pace] will end. Of course, any presidential election injects some uncertainty into the markets and other financial sectors; however, with continued low interest, private equity and OEMs competing for assets, and continued global healthcare growth, the grounds remain fertile for continued M&A. There are so many other topics that were significant this year—from device cybersecurity, wearables and real-time monitoring, the continued roll out of the ACA [Affordable Care Act], value-based healthcare, electronic health records, among others—it is certainly hard to single out just one. Elizabeth Cairns: The most eye-catching finding of 2016 so far has been the collapse in value of closed mergers. At less than $17 billion in the first half of 2016, the aggregate deal value this year is on track to be less than half of that recorded last year. True, 2015 was a record-breaker, with a total value of $127 billion in closed mergers, led by Medtronic’s $50 billion purchase of Covidien. But 2016 is on course to see a lower aggregate value of closed mergers than 2014, too. Still, the number of deals this year looks likely to remain around the same as last year. There is still an appetite for M&A, but the deals are smaller. This ought to be good news for start-ups—if the top medtech companies are spending less time and money on consolidation they will, hopefully, have more resources to dedicate to smaller, technology-based deals. Brian Williams: A number of telemedicine companies have proven to be at the forefront of enabling the transformation of primary care and the expansion of access, without the lengthy training process for clinicians, or the building of additional costly infrastructure. Home diagnostic devices, which perform a multitude of tests in one single device including, heart, ear, and throat exams, to name a few, allow patients to capture the information they need to virtually communicate with their doctor any time of the day. By utilizing a cloud platform, select devices provide secured communication, data repository, analytics and data-sharing between patients, clinicians and healthcare organizations. Telemedicine companies are redefining how medical device and biopharmaceutical manufacturers can support value-based contracts that include post interventional monitoring and support. Barbella: What will capture headlines in the medtech industry in 2017? Bonifacio: In addition to continued M&A and the discussion around pharmaceutical pricing, I think the ACA will have to get some tweaks if it is to continue in any form going forward; while some components seem to working there are still many facets of this law that will need to be modified (there are many details too lengthy to discuss here). Connected and what I’m calling growing consumer health globally will also be at the forefront for 2017 and beyond as I believe we are on the cusp of some of the biggest changes we have seen in decades in our industry and the general healthcare space. Medical devices and technology-driven therapies will push the envelope further than we even dreamed was possible only 10 or 20 years ago. These are indeed challenging times, but exciting times as well. Cairns: It is beginning to look like the big stories of the coming year are to do more with macroeconomic factors rather than medtech-specific issues. The United Kingdom’s decision to leave the European Union will have major repercussions throughout Europe and even beyond. UK-based companies—which include several large multinationals who have based themselves in the UK for tax reasons—could find it harder to attract the skilled scientific workforce on which they rely and already face import pressures from the weakened pound. Companies outside the UK will have to negotiate different laws on regulation and intellectual property, and—partly because there is very little clarity so far on how this might play out—there is anecdotal evidence that some companies are already delaying investment decisions in their UK subsidiaries. The healthcare plans introduced by the new U.S. president will have far-reaching consequences for the U.S. medtech sector. Hillary Clinton has promised to build on and expand the beleaguered Affordable Health Act, whereas Donald Trump has stated that he will repeal it entirely. The medtech industry has fought with some success against the device tax that goes toward funding the ACA, but nonetheless there appears to be overall support for Clinton over Trump, perhaps because she is perceived a safer pair of hands. Williams: Multiple companies are working to develop and deploy technology that expands the reach and capabilities of healthcare without the attendant costs, resulting in a re-balancing of value across healthcare.
|




























