Steve Maylish and Bruce Sargeant , Fusion Biotec09.08.16
A medical instrument development project can involve many different approaches and evaluation of tradeoffs to decrease time to market, which can be complicated. The scenarios range from using commercial off-the-shelf parts to private labeling an existing instrument.
For most 510(k)-approved medical devices, time to market is driven by several factors—development time, time to collect clinical data, and U.S. Food and Drug Administration (FDA) approval time. Time is the enemy in the product launch race. Once lost, it’s difficult or impossible to regain.
In any design project, there are always constraints that must be balanced by project management. In the best circumstances, teams are only able to outperform on one or two of three main goals: cost, quality, or time to market. (Figure 1)
Quality in medical products is not really negotiable, and time to market usually follows a close second behind quality. This often leaves development cost lowest on the priority list. But what if getting to market quickly and keeping costs low is mandated?
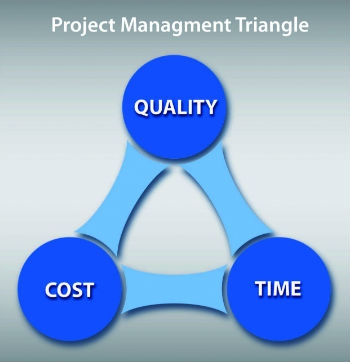
Figure 1
Here again there are tradeoffs, and they’re between two cost components—cost of product development and unit cost. Companies that desire a low unit cost usually have higher development costs, and vice versa. (See last month’s column.) Firms can outperform in all three areas, but the tradeoff then shifts to unit cost and performance rather than development cost (Figure 2).
Before considering cost tradeoffs, consider time to market and the implications of a delayed market launch. For a next-generation instrument design, there may be some cannibalization of existing sales. But for a startup, venture capitalists will say there’s an enormous cost to being late and typically, these costs are not well understood.
For example, to better understand the cost of delay, assume a new product has a 10-year sales cycle and the total revenue over 10 years is $100 million. If the product launch is delayed by two years, or 20 percent, most venture capitalists assume the sales life also declines by that amount. In addition to a new sales life of eight years, they also assume that the peak revenue generated will decline by about half of the delay percentage, or 10 percent. To simplify this, a 20 percent delay is assumed to be a 30 percent loss of revenue—$30 million over the life of the product in this case.
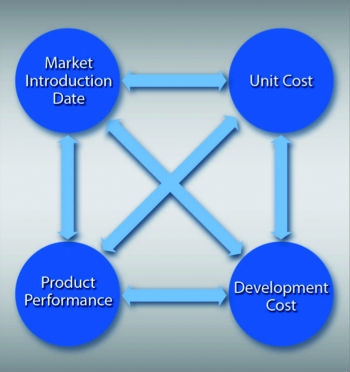
Figure 2
This may or may not end up being the actual case, but the message is loud and clear. There is limited sales life for any medical device and delays have a devastating effect on investment returns.
To decrease time to market or stay on schedule, how does one weigh the options? Or are there ways to minimize their impact?
Figure 3 shows various strategies to decrease time to market. As one moves down the list, time to market, development costs, FDA responsibilities, and technical risks decrease. The tradeoffs are that innovation, ownership, and control of the product decrease, while unit price increases.
One of the best ways to reduce development time is to do less work. From Figure 3, clearly relabeling a finished product is the shortest time to market, while starting a new design in-house may be the longest.
Due to their expertise in a company’s core competence, in-house engineering teams offer great value to medical device companies. If the company is looking to design a new product in-house, it pays to assess the team honestly. Is the team primarily involved in sustaining engineering or new product design? Good design engineers are hungry tigers, constantly being fed new projects. Is the team creatively stale or at the top of its game? Technology is moving faster than ever, and so are the offerings in software and hardware. Does the team have the expertise needed for the contemplated work? Should the team be supplemented by outside domain experts? Domain experts can offer ideas that cross-pollinate various medical applications. This is especially valuable when creating intellectual property and building barriers to entry.

Figure 3
Does the team have the bandwidth to complete the project without competing priorities? Is the company background disposable- or pharmaceutical-centric and is the firm now looking to design and build instruments? How do corporate processes help or hinder the instrument development process? Typically, in-house development is the longest path to a commercial product, and often, it’s more a reflection of the corporate environment than engineering capabilities.
If there’s a disconnect between corporate processes and instrument development, it’s worth the effort to evaluate its effect. Reducing process time and removing obstacles can have a positive impact on in-house design teams. Making a conscious effort to measure and reduce process times can help turn around engineering development time—this requires a company mandate and buy-in from various departments. Monitoring and reducing the time to complete a product requirement document, processing an engineering change order, attending weekly meetings, and completing a design review or creating a mechanical part drawing all can have a dramatic effect.
The tradeoff: unless process barriers can be greatly reduced, the project may take twice as long in-house and outside domain experts may still be needed.
Specialized engineering firms offer specific expertise and seasoned design teams. A good way to reduce cost and time to market for a new device is to take advantage of a specialized design firm’s expertise. The best product development project uses a great team of engineers, a good set of requirements, and a certain amount of team autonomy.
Typically, a specialized design firm will have a culture of moving fast, good team cohesiveness, creative design, and strong cross-pollination of ideas. Other benefits of using a firm include not having to hire and fire engineering staff, ensuring team cohesiveness and having expertise present when needed. A fast-moving development team will fail early and fail often in order to reduce technical risk and prove feasibility. Outsourcing to a specialized firm usually reduces time to market due to a less constrained environment, adds the ability to increase resources as needed, and offers quicker decision making.
This path requires close review if the specialized design firm is looking to incorporate its own technology, provide engineering services for equity, or own patent rights. It can provide a faster path to commercialization, but caution needs to be taken with the tradeoff. Incorporating others’ technology, trading equity, or sharing intellectual property rights for engineering services can cause ownership confusion. In addition, pre-existing subsystems or technology can lock in supply chain and increase unit cost.
An outsourcer should own the work for which it has paid and license any pre-existing technology from the specialized firm. The cost of any pre-existing technology should be known up front. The outsourcer should not pay for development and be required to share intellectual property rights or equity. For pre-existing software, a royalty free, non-exclusive license should be available—if accompanied by paid customized software. This should be available for the permitted purpose of supporting the product throughout its lifetime. Software source code should always be included.
Modifying an existing design is a great strategy for next-generation designs. The fewer changes made, the less development time, risk, and chance of an FDA resubmission. However, if looking to design something new and innovative, a hybrid design may stifle creativity and put too many constraints on the team. When starting with an existing design, the key is to evaluate in detail the amount of changes needed.
An area that is often overlooked is the existing software and how extensively it needs to be rewritten. If the software is too old, poorly written, or fragile, it’s better to completely rewrite it than continue to patch it. With legacy software and multiple contributors, it can be in a state where testing and bug fixes take longer than writing new code. There can also be unsupported or outdated operating systems. This is an area where paying an outside firm to evaluate the software can be extremely valuable.
Where changes are few and functionality similar, modifying an existing design decreases time to market. The tradeoff, again, comes when there are too many changes and starting from scratch would have been simpler and quicker. This is difficult to know at the start, so be very conservative with this approach. The older the original design, the more likely it needs to be scrapped.
Rebranding an OEM instrument can completely eliminate development costs. There may be changes to the labeling, instructions for use, enclosure, and user interface. Be cognizant that the more changes needed to the instrument, the less beneficial it becomes for the OEM. These types of relationships have to be carefully thought out and executed. The key to success on both sides is a supplier agreement that creates a relationship that benefits both companies.
Another rebranding consideration is the FDA implications. If the use case is not the same as the intended use as defined by the instrument’s 510(k), a new submission will likely be necessary. Under European Union directives, the Own Brand Labeler is the legal manufacturer and takes on all legal responsibilities as a manufacturer.
No matter who is manufacturing the device or where it’s sold, as far as the FDA is concerned, the manufacturer of record is the company with its name on the device. (See FDA 21 CFR 820.1 for additional info.) Contract manufacturing companies can use their manufacturing processes and quality systems to fulfill the manufacturing related aspects of FDA 820, but there are other requirements of the manufacturer of record, including maintaining a quality system, reporting customer complaints, and ensuring that the manufacturing process is followed correctly. At least six months is required to work out these processes and make this new system part of the company’s culture.
When relabeling an OEM’s instrument, the first consideration should be branding. An existing OEM instrument may already have brand loyalty in the market, which takes time and money to recreate. If a new application doesn’t require modifications to the existing instrument and the company doesn’t want to be in the instrument business, this may be a great alternative.
If using private label, there’s no new technology or patents created with the instrument. Intellectual property and trade secrets are the foundation of a company’s valuation. Since the U.S. Supreme Court ruling in 2012 of Mayo Collaborative Services v. Prometheus Labs, intellectual property is more difficult to obtain, because measuring a naturally occurring substance in the body with an existing detection method can invalidate the claim.
The largest tradeoff here is product cost. To better understand the increased cost of private label, consider a small diagnostic reader with a cost of goods (materials) at $1,500 per unit and a manufacturing cost of $3,000 (manufacturing margin included). If it’s the company’s instrument, it may sell for $6,000, resulting in a 50 percent gross margin. If it’s supplied by an OEM, it will likely be marked up to cover the cost of development. If the OEM outsources the manufacturing to another firm, the markup will be higher. In this case, it’s very likely that the sale price of the instrument from the OEM to the private labeler is $6,500 or more.
It’s judicious to calculate the total number of instruments to be purchased from the OEM versus the cost of development. At the break-even point and higher, the case for product development becomes stronger. Using an OEM instrument may not be an optimal solution either; performance may also play into the decision.
When seeking to reduce time to market, be aware of the tradeoffs. They can affect many areas of the company and have unintended consequences. If high quality, low development cost, and decreased time to market is selected, intellectual property rights, product cost, or performance may be compromised.
Steve Maylish has been part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences.
Bruce Sargeant is founder and CEO of Fusion Biotec. He has been an innovator and serial entrepreneur in the medical product outsourcing space for over three decades, since co-founding Ocean Scientific in 1980. Most recently, he was CTO at BIT USA, a company created when BIT of Germany purchased Source Scientific and California Medtech.
For most 510(k)-approved medical devices, time to market is driven by several factors—development time, time to collect clinical data, and U.S. Food and Drug Administration (FDA) approval time. Time is the enemy in the product launch race. Once lost, it’s difficult or impossible to regain.
In any design project, there are always constraints that must be balanced by project management. In the best circumstances, teams are only able to outperform on one or two of three main goals: cost, quality, or time to market. (Figure 1)
Quality in medical products is not really negotiable, and time to market usually follows a close second behind quality. This often leaves development cost lowest on the priority list. But what if getting to market quickly and keeping costs low is mandated?

Figure 1
Before considering cost tradeoffs, consider time to market and the implications of a delayed market launch. For a next-generation instrument design, there may be some cannibalization of existing sales. But for a startup, venture capitalists will say there’s an enormous cost to being late and typically, these costs are not well understood.
For example, to better understand the cost of delay, assume a new product has a 10-year sales cycle and the total revenue over 10 years is $100 million. If the product launch is delayed by two years, or 20 percent, most venture capitalists assume the sales life also declines by that amount. In addition to a new sales life of eight years, they also assume that the peak revenue generated will decline by about half of the delay percentage, or 10 percent. To simplify this, a 20 percent delay is assumed to be a 30 percent loss of revenue—$30 million over the life of the product in this case.

Figure 2
To decrease time to market or stay on schedule, how does one weigh the options? Or are there ways to minimize their impact?
Figure 3 shows various strategies to decrease time to market. As one moves down the list, time to market, development costs, FDA responsibilities, and technical risks decrease. The tradeoffs are that innovation, ownership, and control of the product decrease, while unit price increases.
One of the best ways to reduce development time is to do less work. From Figure 3, clearly relabeling a finished product is the shortest time to market, while starting a new design in-house may be the longest.
Due to their expertise in a company’s core competence, in-house engineering teams offer great value to medical device companies. If the company is looking to design a new product in-house, it pays to assess the team honestly. Is the team primarily involved in sustaining engineering or new product design? Good design engineers are hungry tigers, constantly being fed new projects. Is the team creatively stale or at the top of its game? Technology is moving faster than ever, and so are the offerings in software and hardware. Does the team have the expertise needed for the contemplated work? Should the team be supplemented by outside domain experts? Domain experts can offer ideas that cross-pollinate various medical applications. This is especially valuable when creating intellectual property and building barriers to entry.

Figure 3
If there’s a disconnect between corporate processes and instrument development, it’s worth the effort to evaluate its effect. Reducing process time and removing obstacles can have a positive impact on in-house design teams. Making a conscious effort to measure and reduce process times can help turn around engineering development time—this requires a company mandate and buy-in from various departments. Monitoring and reducing the time to complete a product requirement document, processing an engineering change order, attending weekly meetings, and completing a design review or creating a mechanical part drawing all can have a dramatic effect.
The tradeoff: unless process barriers can be greatly reduced, the project may take twice as long in-house and outside domain experts may still be needed.
Specialized engineering firms offer specific expertise and seasoned design teams. A good way to reduce cost and time to market for a new device is to take advantage of a specialized design firm’s expertise. The best product development project uses a great team of engineers, a good set of requirements, and a certain amount of team autonomy.
Typically, a specialized design firm will have a culture of moving fast, good team cohesiveness, creative design, and strong cross-pollination of ideas. Other benefits of using a firm include not having to hire and fire engineering staff, ensuring team cohesiveness and having expertise present when needed. A fast-moving development team will fail early and fail often in order to reduce technical risk and prove feasibility. Outsourcing to a specialized firm usually reduces time to market due to a less constrained environment, adds the ability to increase resources as needed, and offers quicker decision making.
This path requires close review if the specialized design firm is looking to incorporate its own technology, provide engineering services for equity, or own patent rights. It can provide a faster path to commercialization, but caution needs to be taken with the tradeoff. Incorporating others’ technology, trading equity, or sharing intellectual property rights for engineering services can cause ownership confusion. In addition, pre-existing subsystems or technology can lock in supply chain and increase unit cost.
An outsourcer should own the work for which it has paid and license any pre-existing technology from the specialized firm. The cost of any pre-existing technology should be known up front. The outsourcer should not pay for development and be required to share intellectual property rights or equity. For pre-existing software, a royalty free, non-exclusive license should be available—if accompanied by paid customized software. This should be available for the permitted purpose of supporting the product throughout its lifetime. Software source code should always be included.
Modifying an existing design is a great strategy for next-generation designs. The fewer changes made, the less development time, risk, and chance of an FDA resubmission. However, if looking to design something new and innovative, a hybrid design may stifle creativity and put too many constraints on the team. When starting with an existing design, the key is to evaluate in detail the amount of changes needed.
An area that is often overlooked is the existing software and how extensively it needs to be rewritten. If the software is too old, poorly written, or fragile, it’s better to completely rewrite it than continue to patch it. With legacy software and multiple contributors, it can be in a state where testing and bug fixes take longer than writing new code. There can also be unsupported or outdated operating systems. This is an area where paying an outside firm to evaluate the software can be extremely valuable.
Where changes are few and functionality similar, modifying an existing design decreases time to market. The tradeoff, again, comes when there are too many changes and starting from scratch would have been simpler and quicker. This is difficult to know at the start, so be very conservative with this approach. The older the original design, the more likely it needs to be scrapped.
Rebranding an OEM instrument can completely eliminate development costs. There may be changes to the labeling, instructions for use, enclosure, and user interface. Be cognizant that the more changes needed to the instrument, the less beneficial it becomes for the OEM. These types of relationships have to be carefully thought out and executed. The key to success on both sides is a supplier agreement that creates a relationship that benefits both companies.
Another rebranding consideration is the FDA implications. If the use case is not the same as the intended use as defined by the instrument’s 510(k), a new submission will likely be necessary. Under European Union directives, the Own Brand Labeler is the legal manufacturer and takes on all legal responsibilities as a manufacturer.
No matter who is manufacturing the device or where it’s sold, as far as the FDA is concerned, the manufacturer of record is the company with its name on the device. (See FDA 21 CFR 820.1 for additional info.) Contract manufacturing companies can use their manufacturing processes and quality systems to fulfill the manufacturing related aspects of FDA 820, but there are other requirements of the manufacturer of record, including maintaining a quality system, reporting customer complaints, and ensuring that the manufacturing process is followed correctly. At least six months is required to work out these processes and make this new system part of the company’s culture.
When relabeling an OEM’s instrument, the first consideration should be branding. An existing OEM instrument may already have brand loyalty in the market, which takes time and money to recreate. If a new application doesn’t require modifications to the existing instrument and the company doesn’t want to be in the instrument business, this may be a great alternative.
If using private label, there’s no new technology or patents created with the instrument. Intellectual property and trade secrets are the foundation of a company’s valuation. Since the U.S. Supreme Court ruling in 2012 of Mayo Collaborative Services v. Prometheus Labs, intellectual property is more difficult to obtain, because measuring a naturally occurring substance in the body with an existing detection method can invalidate the claim.
The largest tradeoff here is product cost. To better understand the increased cost of private label, consider a small diagnostic reader with a cost of goods (materials) at $1,500 per unit and a manufacturing cost of $3,000 (manufacturing margin included). If it’s the company’s instrument, it may sell for $6,000, resulting in a 50 percent gross margin. If it’s supplied by an OEM, it will likely be marked up to cover the cost of development. If the OEM outsources the manufacturing to another firm, the markup will be higher. In this case, it’s very likely that the sale price of the instrument from the OEM to the private labeler is $6,500 or more.
It’s judicious to calculate the total number of instruments to be purchased from the OEM versus the cost of development. At the break-even point and higher, the case for product development becomes stronger. Using an OEM instrument may not be an optimal solution either; performance may also play into the decision.
When seeking to reduce time to market, be aware of the tradeoffs. They can affect many areas of the company and have unintended consequences. If high quality, low development cost, and decreased time to market is selected, intellectual property rights, product cost, or performance may be compromised.
Steve Maylish has been part of the medical device community for more than 30 years. He is currently chief commercial officer for Fusion Biotec, an Irvine, Calif.-based contract engineering firm that brings together art, science, and engineering to create medical devices. Early in his career, Maylish held positions at Fortune 100 corporations such as Johnson & Johnson, Shiley, Sorin Group, Baxter Healthcare, and Edwards Lifesciences.
Bruce Sargeant is founder and CEO of Fusion Biotec. He has been an innovator and serial entrepreneur in the medical product outsourcing space for over three decades, since co-founding Ocean Scientific in 1980. Most recently, he was CTO at BIT USA, a company created when BIT of Germany purchased Source Scientific and California Medtech.




























