07.26.18
$3.4 Billion
KEY EXECUTIVES:
Michael A. Mussallem, Chairman and CEO
Daveen Chopra, Corp. VP, Surgical Heart Valve Therapies
John P. McGrath, Ph.D., Corp. VP, Quality, Regulatory, Clinical
Joseph Nuzzolese, Corp. VP, Global Supply Chain
Stanton J. Rowe, Corp. VP, Advanced Technology and Chief Scientific Officer
Catherine M. Szyman, Corp. VP, Critical Care
Scott B. Ullem, Corp. VP, CFO
Larry L. Wood, Corp. VP, Transcatheter Heart Valves
Bernard J. Zovighian, Corp. VP, Transcatheter Mitral and Tricuspid Therapies
NO. OF EMPLOYEES: 12,200
GLOBAL HEADQUARTERS: Irvine, Calif.
Even at 66 years of age, James Garrett led a very active lifestyle. His passion for rock climbing resulted in him being credited with more than 300 “first ascents,” pioneering climbing routes all over the world. In his travels as a flight nurse, he also witnessed many medical needs in developing countries. One that particularly interested him was the battle against cataracts. He began volunteering with the Himalayan Cataract Project where he mentors and works with local paramedical staff during high-volume cataract surgery campaigns to help restore sight to those with little access to eye care around the world.
On an average evening in 2017, while on the couch with his physician wife, all of that came to a sudden and frightening halt.
“I had my head and my ear against his chest,” said James’ wife, Franziska Garrett, M.D. She then sprang up suddenly and exclaimed that she heard a really loud murmur. She continued, “We knew we had to see a cardiologist right away. I was definitely shocked.”
The cardiologist noted James’ aortic valve was a congenitally malformed bicuspid valve, diagnosed him with aortic stenosis, and asked with urgency how soon could they schedule James for open-heart surgery.
As would be understandable, James wasn’t on board with the prospect of open-heart surgery.
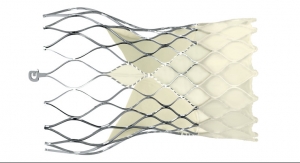
Oddly fortunate, during one of his trips, James contracted a viral respiratory illness that created questions about the safety of performing an open-heart procedure on him. As a result, James Harkness, M.D., interventional cardiologist and Stephen Clayson, M.D., cardiothoracic surgeon, both of Intermountain Medical Center Heart Institute in Salt Lake City, Utah, began to explore other treatment options. TAVR (transcatheter aortic valve replacement) was selected as the best alternative for him. The Edwards SAPIEN 3 transcather heart valve was the specifically chosen solution.
As a result of the TAVR technique, physicians were able to perform the procedure with James under conscious sedation, meaning he would not be under general anesthesia, which was important given his respiratory condition.
A few weeks after placement of the replacement valve, James felt like “himself” again and his lungs were healing as well. He has continued his work in the fight against cataracts in regions around the world and he and his wife are very grateful to be able to do so.
Unfortunately, the health battle patients like James face requiring the placement of a TAVR device isn’t the only fight Edwards has been associated with involving the technology. In 2017, the company faced ongoing legal challenges regarding the validity of several patents in a dispute with Boston Scientific.
In March 2017, a U.K. patent court and a German patent court both issued initial decisions that stated one of Boston Scientific’s patents asserted against Edwards’ patent was invalid while another was deemed valid and infringed. A year later, the European Patent Office sided with Boston Scientific, along with several other opponents, in its dispute of Edwards Lifesciences’ European patent EP 2,399,550, which resulted in a revocation of the patent. This action harkened back to the German court decision, which found Boston Scientific infringed upon that patent. The patent dispute is ongoing.
Meanwhile, in the United States in March 2018, the U.S. Patent and Trademark Office decided in Edwards’ favor in an Inter Partes Review of Boston Scientific’s U.S. transcatheter heart valve patent, number 8,992,608. All claims of the ‘608 patent asserted against Edwards were determined to be invalid. Similar to the European case, the U.S. dispute is ongoing as well.
As can be observed in the anecdotal story involving James Garrett, TAVR represents a new, novel therapeutic approach to treat a damaged heart valve. As such, it’s no surprise to see medtech companies fight over the rights to the patents involved with the technology. It could result in a significant reward for the “winner” in licensing agreements and/or market share.
Certainly, Edwards is well aware of the potential of the technology. The company already saw an impressive 16 percent increase in 2017’s net sales ($3.4 billion) over 2016’s just-shy $3 billion total. Further, the company has enjoyed steady growth since at least 2013 when it posted $2 billion in sales, but noted in its 2017 annual report it has experienced 10 years of double-digit adjusted sales growth. With a focus on cardiovascular disease, the company approaches its treatment options for healthcare by way of three separate divisions—Transcatheter Heart Valve Therapy, Surgical Heart Valve Therapy, and Critical Care.
The Transcatheter Heart Valve Therapy unit is helmed by two major products for Edwards, the SAPIEN XT and SAPIEN 3 transcatheter aortic heart valves and their respective delivery systems. The segment contributes a majority number to the company’s sales total, delivering 59 percent in 2017 (a significant increase compared to 47 percent just two years prior). That translates to just over $2 billion in 2017 net sales—an almost 25 percent increase over 2016, which had experienced a 38 percent rise over its prior year.
The next largest unit in net sales in 2017 was Surgical Heart Valve Therapy. This unit’s signature product is the Carpentier-Edwards PERIMOUNT pericardial valve platform, including the line of PERIMOUNT Magna Ease pericardial valves for aortic and mitral surgical valve replacement. Contributing $807 million in 2017, the segment enjoyed a 4.2 percent increase over the prior year, which was a welcome change for the company compared to its 2016 total when the unit’s sales shrank 1.3 percent from 2015.
Accounting for the remainder is Critical Care, comprised of hemodynamic monitoring systems used to measure a patient’s heart function and fluid status in surgical and intensive care settings. Edwards offers a selection of products for this space, including the minimally invasive FloTrac system and the noninvasive ClearSight system. It saw sales of $601 million in 2017, which was a bump up from 2016 by 7.3 percent.
Edward’s biggest market is the United States, where it achieved sales of more than 1.9 billion in fiscal 2017. Internationally, it took in $1.5 billion, with Europe ($831 million) and Japan ($350 million) reflecting the lion’s share of that total.
Not satisfied to rest on its laurels as it looks to the future, Edwards made two complementary acquisitions in 2017 to help ensure its growth remains positive. In the first month of its 2017 fiscal year, Edwards completed a previously announced purchase. In November 2016, the firm revealed it would be buying Valtech Cardio Ltd. for $340 million, with the potential for up to $350 million in pre-specified milestone driven payments over the next 10 years. The Israeli firm developed the Cardioband system, which is used for transcatheter repair of the mitral and tricuspid valves.
“We look forward to the Valtech team joining Edwards. We believe their knowledge, experience, and the Cardioband technology are valuable additions to Edwards,” Michael A. Mussallem, Edwards’ chairman and CEO, said in a news release declaring the close of the transaction. “This therapy has the potential to be a breakthrough structural heart therapy to help many patients in desperate need, and we look forward to gaining valuable insights from its commercial use in Europe.”
The device still awaits FDA clearance in the United States.
Bookending the fiscal year with the Valtech acquisition, Edwards announced the December 1 closing of its acquisition of Harpoon Medical on December 6. Harpoon’s focus was on the development of technology to enable beating-heart repair for degenerative mitral regurgitation. The purchase price was $100 million in cash at the close, with the potential for an additional $150 million if certain milestones are met within the following 10 years. According to Edwards, the system is designed to facilitate echo-guided repair of mitral valve regurgitation by stabilizing the prolapsed mitral valve leaflet to restore proper coaptation and valve function.
Regarding the acquisition of Harpoon, Bernard Zovighian, Edwards’ then corporate vice president of surgical heart valve therapy, said, “The unique beating-heart repair procedure for mitral valve patients complements Edwards’ comprehensive portfolio of treatments for structural heart disease, and reinforces our commitment to innovation in cardiac surgery.”
KEY EXECUTIVES:
Michael A. Mussallem, Chairman and CEO
Daveen Chopra, Corp. VP, Surgical Heart Valve Therapies
John P. McGrath, Ph.D., Corp. VP, Quality, Regulatory, Clinical
Joseph Nuzzolese, Corp. VP, Global Supply Chain
Stanton J. Rowe, Corp. VP, Advanced Technology and Chief Scientific Officer
Catherine M. Szyman, Corp. VP, Critical Care
Scott B. Ullem, Corp. VP, CFO
Larry L. Wood, Corp. VP, Transcatheter Heart Valves
Bernard J. Zovighian, Corp. VP, Transcatheter Mitral and Tricuspid Therapies
NO. OF EMPLOYEES: 12,200
GLOBAL HEADQUARTERS: Irvine, Calif.
Even at 66 years of age, James Garrett led a very active lifestyle. His passion for rock climbing resulted in him being credited with more than 300 “first ascents,” pioneering climbing routes all over the world. In his travels as a flight nurse, he also witnessed many medical needs in developing countries. One that particularly interested him was the battle against cataracts. He began volunteering with the Himalayan Cataract Project where he mentors and works with local paramedical staff during high-volume cataract surgery campaigns to help restore sight to those with little access to eye care around the world.
On an average evening in 2017, while on the couch with his physician wife, all of that came to a sudden and frightening halt.
“I had my head and my ear against his chest,” said James’ wife, Franziska Garrett, M.D. She then sprang up suddenly and exclaimed that she heard a really loud murmur. She continued, “We knew we had to see a cardiologist right away. I was definitely shocked.”
The cardiologist noted James’ aortic valve was a congenitally malformed bicuspid valve, diagnosed him with aortic stenosis, and asked with urgency how soon could they schedule James for open-heart surgery.
As would be understandable, James wasn’t on board with the prospect of open-heart surgery.
Oddly fortunate, during one of his trips, James contracted a viral respiratory illness that created questions about the safety of performing an open-heart procedure on him. As a result, James Harkness, M.D., interventional cardiologist and Stephen Clayson, M.D., cardiothoracic surgeon, both of Intermountain Medical Center Heart Institute in Salt Lake City, Utah, began to explore other treatment options. TAVR (transcatheter aortic valve replacement) was selected as the best alternative for him. The Edwards SAPIEN 3 transcather heart valve was the specifically chosen solution.
As a result of the TAVR technique, physicians were able to perform the procedure with James under conscious sedation, meaning he would not be under general anesthesia, which was important given his respiratory condition.
A few weeks after placement of the replacement valve, James felt like “himself” again and his lungs were healing as well. He has continued his work in the fight against cataracts in regions around the world and he and his wife are very grateful to be able to do so.
Unfortunately, the health battle patients like James face requiring the placement of a TAVR device isn’t the only fight Edwards has been associated with involving the technology. In 2017, the company faced ongoing legal challenges regarding the validity of several patents in a dispute with Boston Scientific.
In March 2017, a U.K. patent court and a German patent court both issued initial decisions that stated one of Boston Scientific’s patents asserted against Edwards’ patent was invalid while another was deemed valid and infringed. A year later, the European Patent Office sided with Boston Scientific, along with several other opponents, in its dispute of Edwards Lifesciences’ European patent EP 2,399,550, which resulted in a revocation of the patent. This action harkened back to the German court decision, which found Boston Scientific infringed upon that patent. The patent dispute is ongoing.
Meanwhile, in the United States in March 2018, the U.S. Patent and Trademark Office decided in Edwards’ favor in an Inter Partes Review of Boston Scientific’s U.S. transcatheter heart valve patent, number 8,992,608. All claims of the ‘608 patent asserted against Edwards were determined to be invalid. Similar to the European case, the U.S. dispute is ongoing as well.
As can be observed in the anecdotal story involving James Garrett, TAVR represents a new, novel therapeutic approach to treat a damaged heart valve. As such, it’s no surprise to see medtech companies fight over the rights to the patents involved with the technology. It could result in a significant reward for the “winner” in licensing agreements and/or market share.
Certainly, Edwards is well aware of the potential of the technology. The company already saw an impressive 16 percent increase in 2017’s net sales ($3.4 billion) over 2016’s just-shy $3 billion total. Further, the company has enjoyed steady growth since at least 2013 when it posted $2 billion in sales, but noted in its 2017 annual report it has experienced 10 years of double-digit adjusted sales growth. With a focus on cardiovascular disease, the company approaches its treatment options for healthcare by way of three separate divisions—Transcatheter Heart Valve Therapy, Surgical Heart Valve Therapy, and Critical Care.
The Transcatheter Heart Valve Therapy unit is helmed by two major products for Edwards, the SAPIEN XT and SAPIEN 3 transcatheter aortic heart valves and their respective delivery systems. The segment contributes a majority number to the company’s sales total, delivering 59 percent in 2017 (a significant increase compared to 47 percent just two years prior). That translates to just over $2 billion in 2017 net sales—an almost 25 percent increase over 2016, which had experienced a 38 percent rise over its prior year.
The next largest unit in net sales in 2017 was Surgical Heart Valve Therapy. This unit’s signature product is the Carpentier-Edwards PERIMOUNT pericardial valve platform, including the line of PERIMOUNT Magna Ease pericardial valves for aortic and mitral surgical valve replacement. Contributing $807 million in 2017, the segment enjoyed a 4.2 percent increase over the prior year, which was a welcome change for the company compared to its 2016 total when the unit’s sales shrank 1.3 percent from 2015.
Accounting for the remainder is Critical Care, comprised of hemodynamic monitoring systems used to measure a patient’s heart function and fluid status in surgical and intensive care settings. Edwards offers a selection of products for this space, including the minimally invasive FloTrac system and the noninvasive ClearSight system. It saw sales of $601 million in 2017, which was a bump up from 2016 by 7.3 percent.
Edward’s biggest market is the United States, where it achieved sales of more than 1.9 billion in fiscal 2017. Internationally, it took in $1.5 billion, with Europe ($831 million) and Japan ($350 million) reflecting the lion’s share of that total.
Not satisfied to rest on its laurels as it looks to the future, Edwards made two complementary acquisitions in 2017 to help ensure its growth remains positive. In the first month of its 2017 fiscal year, Edwards completed a previously announced purchase. In November 2016, the firm revealed it would be buying Valtech Cardio Ltd. for $340 million, with the potential for up to $350 million in pre-specified milestone driven payments over the next 10 years. The Israeli firm developed the Cardioband system, which is used for transcatheter repair of the mitral and tricuspid valves.
“We look forward to the Valtech team joining Edwards. We believe their knowledge, experience, and the Cardioband technology are valuable additions to Edwards,” Michael A. Mussallem, Edwards’ chairman and CEO, said in a news release declaring the close of the transaction. “This therapy has the potential to be a breakthrough structural heart therapy to help many patients in desperate need, and we look forward to gaining valuable insights from its commercial use in Europe.”
The device still awaits FDA clearance in the United States.
Bookending the fiscal year with the Valtech acquisition, Edwards announced the December 1 closing of its acquisition of Harpoon Medical on December 6. Harpoon’s focus was on the development of technology to enable beating-heart repair for degenerative mitral regurgitation. The purchase price was $100 million in cash at the close, with the potential for an additional $150 million if certain milestones are met within the following 10 years. According to Edwards, the system is designed to facilitate echo-guided repair of mitral valve regurgitation by stabilizing the prolapsed mitral valve leaflet to restore proper coaptation and valve function.
Regarding the acquisition of Harpoon, Bernard Zovighian, Edwards’ then corporate vice president of surgical heart valve therapy, said, “The unique beating-heart repair procedure for mitral valve patients complements Edwards’ comprehensive portfolio of treatments for structural heart disease, and reinforces our commitment to innovation in cardiac surgery.”