The Big Picture
Medical devices—including medical wearables such as smartwatches, fitness trackers, ECG monitors, blood pressure monitors, and biosensors—obtain and transmit data including ECG, heart rate, blood pressure, respiration rate, blood oxygen saturation, blood glucose, skin perspiration, body temperature, and motion evaluation. Suppose a device were to fail after exposure to something as common as rain, steam from a shower, sweat, or a common accident like a drop into a swimming pool. In these cases, critical data could be lost, impacting the availability of medical information. This issue could cause consumers and healthcare practitioners to lose trust in medical device products, increase repairs or warranty claims, and possibly lead to a recall.
In an attempt to put users at ease regarding the aforementioned hazards, many medical wearable device manufacturers employ industry standards including Ingress Protection (IP) (e.g., IPX7, IP65, etc.), UL, USP, and ASTM to name a few. Due to the standard limitations, Ingress Protection alone is insufficient as a way to provide confidence in a device's ability to brave the elements, let alone stand up to common usage.
The Story of IP Standards
Waterproof electronics are often a necessity of intended product use and certainly an active topic of user conversations with the word "waterproof" frequently appearing in marketing messaging. Still, electronics often fall short of delivering promised protection from liquid submersion, mainly due to the utilization of traditional product designs, including mechanical seals and gaskets.
The International Organization for Standardization (ISO) attempted to clear the confusion by replacing the word "waterproof" with "water-resistant." This occurred in 1990 when ISO issued a new standard—ISO 2281—for water-resistance. After various lawsuits were placed against brands concerning inadequate water-resistance, ISO once more attempted to clarify again in 2010, replacing the ISO 2281 standard with ISO 22810 to update the framework.
In doing so, ISO effectively created a measuring stick for product protection called the "Ingress Protection Standards" to inform consumers about how protected their devices were against water. The protection gauged by IP standards measures how well protection keeps contaminants, corrosives, and other hazards out of medical devices. The standards are denoted as IP, followed by a two-digit integer. The first digit represents protection against particle or dust ingress, while the second number represents protection against moisture ingress. The higher the number, the more protected the device is.
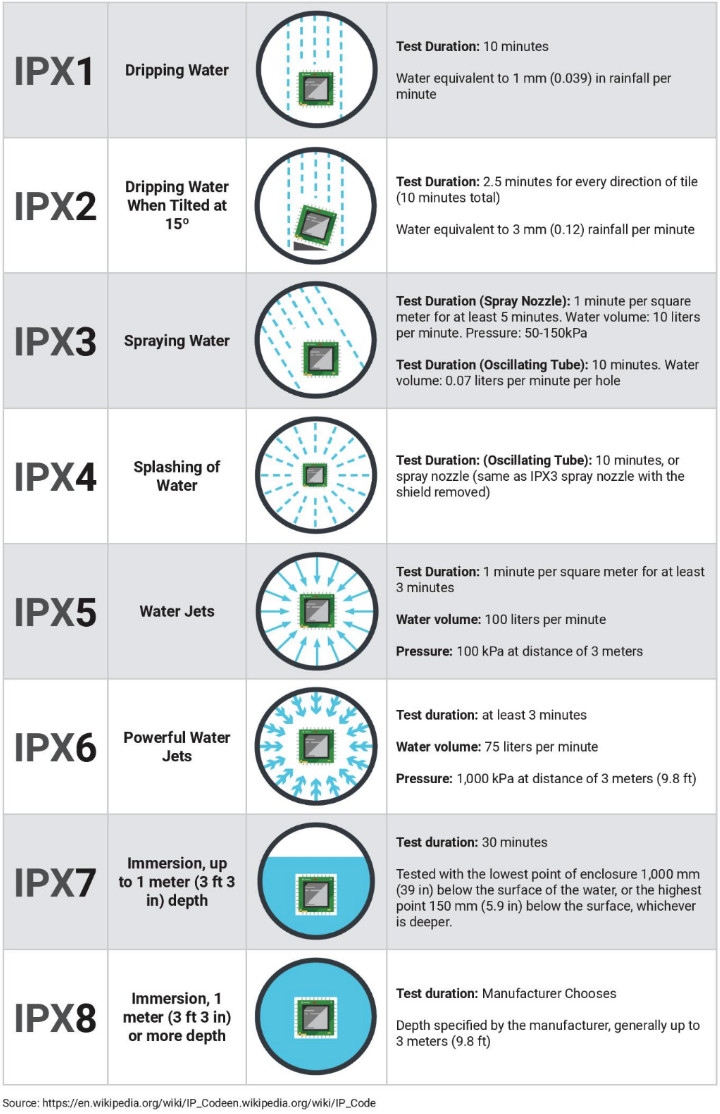
Why IP Standards Are Insufficient
Although commonly used for medical devices such as wearables and hearing aids, IP Standards are insufficient to measure actual product protection. User confusion results from IP Standard testing requirements not serving as a reasonable proxy for actual use.
IP Standard Testing Conditions
- Freshwater only
- Relative humidity range: 20% to 75%
- Air pressure range: 86 KPa to 106 Kpa
- Recommended temperature range: 13°C to 35°C
Issues with IP Standard Testing Conditions
The challenges with IP Standard testing conditions include:
- Products only tested based on exposure to clean water, as opposed to moisture encountered in real life, such as swimming pools, tap water, bodily fluids, saltwater, toilets, or bathtubs.
- Limitations on crucial test parameters (e.g., depth, duration of submersion or liquid exposure, pressure, or temperature ranges) do not approximate real-life usage.
- Only new, out-of-the-box devices that do not have to be powered on during the trial are evaluated.
Setting the New Protection Standard
Thin-film solutions and nanocoatings go beyond IP ratings, offering superior protection against virtually every common environmental threat a medical device is bound to encounter: corrosives, humidity, saltwater, ionized tap water, pollutants, blood, sweat, cleaning fluids, and medicine, to name a few. These coatings take a different approach to protection. Rather than seeking to prevent ingress, thin-film solutions such as Parylene and nanocoatings are applied directly to circuitry inside medical devices, forming a significant barrier from environmental threats.
This type of protection sets a new standard—one that exists beyond the lab's confines and intuitively works in real-life scenarios. For example, Parylene—a thin-film solution—has exceptional anti-corrosive properties that protect against all liquids and corrosives.
|
Material |
WVTR (g·mil)/(100in2·day) |
|
Parylene (Thin Film) |
>0.21 |
|
Epoxy |
6.6 |
|
Urethane |
20.2 |
The water vapor transmission rate (WVTR) measures how fast liquids can penetrate a protective barrier and reach the surface. If water can't reach the surface, corrosion can't occur. Therefore, very low WVTR is critical.
In addition, thin-film materials such as Parylene have exceptional dielectric, thermal, and mechanical properties. A layer of Parylene is thinner than a single strand of human hair, which makes it an ideal alternative to seals and gaskets, which have significantly more mass, adding bulk and weight to miniaturized medical wearables and devices.
Nanocoatings measure even thinner, affording protection at the nanoscale, measured at 10-9. These protective coatings can be composed of various materials to be custom-tailored to protect against common threats to medical devices. They are typically transparent, functionalized films that, depending on the material and process, provide benefits such as hydrophobicity, oleophobicity, corrosion-resistance, wear-resistance, and more.
Benefits of Thin-Film Solutions and Nanocoatings
The contention that sparked with IP ratings was that electronic devices could not deliver what they claimed—fully waterproofed circuitry that would maintain operation and functionality in real-life scenarios. With thin-film solutions and nanocoatings, MDMs, engineers, and designers can make these claims and deliver the promised protection.
When medical devices operate the way they are expected to, medical device OEMs experience cost-avoidance from reduced repairs, refunds, and warranty claims. This cost-avoidance can represent significant savings, as repairs can often cost hundreds or even thousands of dollars. Up to 40 percent of online purchases get returned, with the inability to meet expectations cited as a primary reason, let alone warranty claims that can cost OEMs millions per year.
Avoiding product recalls is another benefit of protecting electronics with the average cost of a recall being $12 million. After a recall, almost 15 percent of customers indicate they would refrain from buying the recalled product again, and 21 percent would not accept any brand associated with the manufacturer. Thin-film solutions and nanocoatings help ensure medical devices work as designed, well beyond their useful life.
Conclusion
IP standard claims are a good start in measuring product protection but not the ultimate gauge for reliable functionality outside of the lab. MDMs, engineers, and designers should consider different protection options in place of seals and gaskets to ensure their devices remain fully functional and reliable. Nanocoatings and thin-film solutions offer a promising alternative.
Zsolt Pulai joined HZO in 2017 as vice president of engineering. Prior to HZO, Pulai was the CEO of ZPL Technologies, a manufacturing system deployment firm that proved critical in the development and deployment of proprietary HZO protective layering equipment into various manufacturing processes. Pulai’s equipment experience includes 10+ years designing deposition equipment for the thin-film solar industry. Prior to this, Pulai was a software engineering consultant for manufacturing control systems and mechanical solutions, including tool-and-die deliverables for companies including Audi and Phillips. Pulai holds degrees in Software Engineering, Embedded Programming and Factory Automation Engineering from Szechenyi University in Gyor, Hungary. He has an MBA Degree in Business Strategy from the MBA Institute and is a Scrum Master Certified Engineer.



















