Michael Barbella, Managing Editor11.17.20
After losing four babies, Jamie McDonald was overjoyed to learn she was expecting twins. But her happiness abruptly ended when she gave birth to the tiny pair at 24 weeks. “We felt pretty positive we were never going to bring them home,” she recounted to “The Doctors” syndicated talk show.
McDonald’s babies—Everly and Maverick—weighed 1.9 pounds at birth and both were diagnosed with patent ductus arteriosus (PDA), a potentially life-threatening congenital heart condition triggered by an opening between two cardiac blood vessels. The sixth most common defect, PDA occurs in 5-10 percent of all children born with congenital heart disease, and is twice as likely to occur in girls than boys.
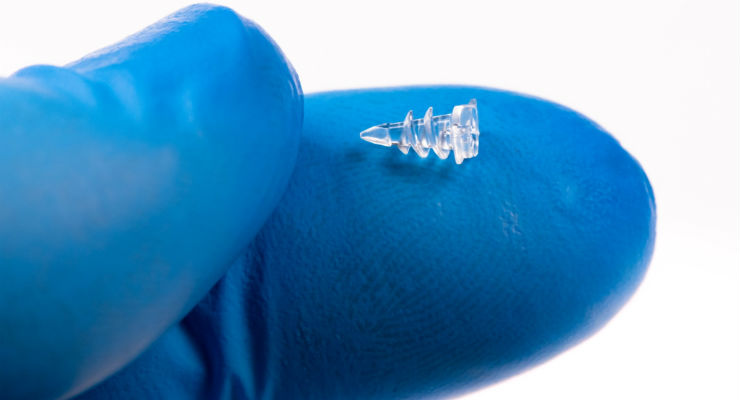
The condition is treatable with therapy, minimally-invasive catheter-based interventions, and minimally-invasive surgical solutions. Doctors chose the latter option for Everly and Maverick, implanting a pea-sized wire mesh device between their pulmonary arteries and aortas to prevent blood from mixing and straining their tiny hearts and lungs.
The device, made by Abbott Laboratories, is inserted through a small leg incision and guided to the afflicted cardiac vessels. Manufacturing such a diminutive object most certainly entailed micromolding, a type of injection molding that produces extremely tiny parts, often with micron tolerances. The process uses special equipment that can produce intricate designs and details.
Medical Product Outsourcing’s October feature, “Little Big Parts,” addresses the latest trends and challenges shaping the fast-growing medical micromolding market. Donna Bibber, vice president of Business Development; Brent Hahn, director of global sales; and Wayne Shakal, vice president of operations at Isometric Micro Molding Inc., were among the various experts interviewed for the story. Their full input is provided in the following Q&A.
Michael Barbella: What market forces are fueling the need for micromolding technology and services?
Donna Bibber: In the past few decades, traditional markets for micromolding have primarily been driven by minimally invasive surgery, which is still a main driver. In recent years, however, drug delivery devices such as aspirators, sub-Q injectors, dermal patches, implantable sensors, and wearable smart devices have driven new products requiring high volume micro molded components and automated assemblies.
Barbella: How are minimally invasive and point-of-care applications affecting micromolded device design and development?
Bibber: MIS and POC device design have flipped the molding industry upside down as enabling technologies to these devices are most often the smallest components with the most challenging requirements of single micron tolerances. This has opened the door for micro molding companies to address these needs with the likes of the biopharma and pharma OEMs who traditionally partner with conventional contract manufacturing companies. With the number of failures going this traditional route, and the corresponding dollars spent on conventional contract manufacturers that don’t deal in single microns on a daily basis, micro molders and vertically integrated micro automated manufacturers like Isometric, have been selected to mitigate these scalability risks that bring MIS and POC miniaturized devices to market faster with much higher precision.
Barbella: What material advancements are currently impacting micromolding capabilities?
Brent Hahn: Very few, however Isometric Micro Molding has created an innovative way to trial different resins at 0.001” – 0.008” wall thicknesses and with all gate types to best gather empirical data to best design and build micro molds and provide our customers with critical feedback. Traditional mold flow software and resin data sheets are optimized with traditional gates and larger part sizes. Many of the parts we mold are smaller than the diameter of the gate being used to create the data engineers can access. Working with Isometric Micro Molding, we can take the current resins available and create innovative micro feature and micro sized solutions.
Barbella: What are customers demanding or expecting in their micromolded products?
Hahn: We continue to hear from customers that their previous molder could make the part once or twice, but not consistently and not in a scalable fashion. Molding a micro featured or micro sized parts doesn’t mean 1-cavity solutions. Multi-cavity solutions, especially for high volume applications, are critical to our customers’ success and one of our core competencies. Another critical demand is being able to have automated assembly after the part is molded. Keeping the part orientation and eliminating damage to these fine features and delicate parts is vital when assembling it with another molded component, metal, adhesive, welding, coating, and many other post molding operations. Being able to pick these type of parts out of the micro molds, keep orientation, and protect the part from damage during this process is an art form all upon itself and something that adds value for our customers.
Barbella: What are the latest innovations in micromolding technology?
Wayne Shakal: From a molding perspective most of the new developments in micro-molding have been incremental improvements in existing technologies. For example, micro-molding machines continue to increase injection speeds and pressures. Close-looped response times are also getting faster and this is improving accuracy which is critical for tight tolerance micro components. To date, we are not aware of any industry altering innovations, but we are aware of the need and currently have multiple R&D efforts in process.
Barbella: How is the need for smaller, more complex medical devices/components challenging micromolding suppliers and providers?
Shakal: The development, FDA approval, and commercialization of micro-sized medical devices is more common than ever. This trend is naturally driving micro-molders to continually increase the level of what can be manufactured. These new product needs are continuously requiring that we strive for and achieve new technology milestones. What was state-of-the-art yesterday might not be today. Feature sizes are getting so small that simply injecting faster or at higher temps isn’t enough. We are required to innovate and develop new processes and methods that are outside of what is considered standard injection molding. Along with the challenge of molding these micro components comes the challenge of being able to handle these components to prevent damage or not impart bio-burden. This requires robotics and custom part retrieval systems for every micro-molded component. Inspection is also an area that can be extremely challenging. Specialty metrology equipment such as micro CT scanners or laser microscopes are required, and often, customization of their normal intended use is required in order to develop a metrology process that is capable of meeting gauge R&R requirements on a tolerance that may be less the 5 microns. This expertise is accomplished through significant trial and error testing and is the enabler that allows us to succeed where others might fail. Lastly, as complicated and challenging as the micro-molding might be, the assembly of these devices can be even more complex and difficult to scale to high volume manufacturing. We are continuously having to push the limits of what is available with current vision and motion control and it is critical that we are able to design and build these systems in-house so that we control all aspects of the system. We have applications requiring 1 micron (.00004”) positional accuracy in the placement of components and this is a combination of tooling, vision, and motion precision. This can only work if you control all aspects of what is required.
Barbella: What factors must be taken into consideration in designing tooling for micromolded parts?
Shakal: A tool designer must have a clear understanding of what is possible with micro-machining in order to properly design a mold that is manufacturable. For example, it is not uncommon for us to see part features that are only a few thousandths in size. The designer must know what machining method will be optimal to put the feature into the tool steel and whether or not there are limitations with cutter reach, cutter diameter, electrode overburn, accuracy requirements, etc. Often these limitations require separating this tool steel out from the rest of the cavity and manufacturing it as a stand-alone piece. Knowing that this is required adds complexity in fitting the componentry together without giving up positional accuracy of a micron. Also, due to the extreme injection speeds and extremely small fill volumes, the molds must be able to vent properly. This is important in any mold but it is exceedingly more difficult to achieve in many micro applications. The difference from failure and success is once again at the micron level. A 1-2 micron difference in vent depth can be the difference between a part that will not fill and a part that has too much flash. It takes a pain staking attention to detail, beginning at design and carried all the way through for these applications to be a success.
Barbella: Should micromolding tooling design be outsourced? Why or why not?
Shakal: From experience, we feel very strongly that in order to provide successful results for a true micro application that is not only micro in size but also has micron level tolerances requires the supplier to be vertically integrated. Tooling is one of the key enablers to the success of a micro program and we don’t believe that we could be timely and effective with outsourced tooling. There is simply too much interaction required between the tooling and development molding teams to do this efficiently with outside resources. It is also important from our perspective that this vertical integration not only exists between tooling and molding but also part handling automation, all metrology, and automated component assembly. The tolerance stack-ups are often so tight that we must control every aspect of manufacturing. It takes considerable resources and time to build up this diverse skill set but it is critical to our customer’s success that we be able to provide this level of service.
Barbella: What new micromolding technologies (if any) are on the horizon?
Shakal: Our experience has presented us with many problems and limitations in the industry. We currently have numerous R&D efforts in process that we are planning to offer our customers in the near future. Some of these solutions include ‘gateless’ parts and the ability to fill long thin wall micro parts. We are also an early adopter of ultrasonic injection molding and are currently working with the developer of this technology on some very demanding applications where micro molding is not possible due to the limited pressure and heat that the finished product can be exposed to. This technology has also shown to have the potential of filling smaller features and longer flow lengths with significantly less pressure.
McDonald’s babies—Everly and Maverick—weighed 1.9 pounds at birth and both were diagnosed with patent ductus arteriosus (PDA), a potentially life-threatening congenital heart condition triggered by an opening between two cardiac blood vessels. The sixth most common defect, PDA occurs in 5-10 percent of all children born with congenital heart disease, and is twice as likely to occur in girls than boys.
The condition is treatable with therapy, minimally-invasive catheter-based interventions, and minimally-invasive surgical solutions. Doctors chose the latter option for Everly and Maverick, implanting a pea-sized wire mesh device between their pulmonary arteries and aortas to prevent blood from mixing and straining their tiny hearts and lungs.
The device, made by Abbott Laboratories, is inserted through a small leg incision and guided to the afflicted cardiac vessels. Manufacturing such a diminutive object most certainly entailed micromolding, a type of injection molding that produces extremely tiny parts, often with micron tolerances. The process uses special equipment that can produce intricate designs and details.
Medical Product Outsourcing’s October feature, “Little Big Parts,” addresses the latest trends and challenges shaping the fast-growing medical micromolding market. Donna Bibber, vice president of Business Development; Brent Hahn, director of global sales; and Wayne Shakal, vice president of operations at Isometric Micro Molding Inc., were among the various experts interviewed for the story. Their full input is provided in the following Q&A.
Michael Barbella: What market forces are fueling the need for micromolding technology and services?
Donna Bibber: In the past few decades, traditional markets for micromolding have primarily been driven by minimally invasive surgery, which is still a main driver. In recent years, however, drug delivery devices such as aspirators, sub-Q injectors, dermal patches, implantable sensors, and wearable smart devices have driven new products requiring high volume micro molded components and automated assemblies.
Barbella: How are minimally invasive and point-of-care applications affecting micromolded device design and development?
Bibber: MIS and POC device design have flipped the molding industry upside down as enabling technologies to these devices are most often the smallest components with the most challenging requirements of single micron tolerances. This has opened the door for micro molding companies to address these needs with the likes of the biopharma and pharma OEMs who traditionally partner with conventional contract manufacturing companies. With the number of failures going this traditional route, and the corresponding dollars spent on conventional contract manufacturers that don’t deal in single microns on a daily basis, micro molders and vertically integrated micro automated manufacturers like Isometric, have been selected to mitigate these scalability risks that bring MIS and POC miniaturized devices to market faster with much higher precision.
Barbella: What material advancements are currently impacting micromolding capabilities?
Brent Hahn: Very few, however Isometric Micro Molding has created an innovative way to trial different resins at 0.001” – 0.008” wall thicknesses and with all gate types to best gather empirical data to best design and build micro molds and provide our customers with critical feedback. Traditional mold flow software and resin data sheets are optimized with traditional gates and larger part sizes. Many of the parts we mold are smaller than the diameter of the gate being used to create the data engineers can access. Working with Isometric Micro Molding, we can take the current resins available and create innovative micro feature and micro sized solutions.
Barbella: What are customers demanding or expecting in their micromolded products?
Hahn: We continue to hear from customers that their previous molder could make the part once or twice, but not consistently and not in a scalable fashion. Molding a micro featured or micro sized parts doesn’t mean 1-cavity solutions. Multi-cavity solutions, especially for high volume applications, are critical to our customers’ success and one of our core competencies. Another critical demand is being able to have automated assembly after the part is molded. Keeping the part orientation and eliminating damage to these fine features and delicate parts is vital when assembling it with another molded component, metal, adhesive, welding, coating, and many other post molding operations. Being able to pick these type of parts out of the micro molds, keep orientation, and protect the part from damage during this process is an art form all upon itself and something that adds value for our customers.
Barbella: What are the latest innovations in micromolding technology?
Wayne Shakal: From a molding perspective most of the new developments in micro-molding have been incremental improvements in existing technologies. For example, micro-molding machines continue to increase injection speeds and pressures. Close-looped response times are also getting faster and this is improving accuracy which is critical for tight tolerance micro components. To date, we are not aware of any industry altering innovations, but we are aware of the need and currently have multiple R&D efforts in process.
Barbella: How is the need for smaller, more complex medical devices/components challenging micromolding suppliers and providers?
Shakal: The development, FDA approval, and commercialization of micro-sized medical devices is more common than ever. This trend is naturally driving micro-molders to continually increase the level of what can be manufactured. These new product needs are continuously requiring that we strive for and achieve new technology milestones. What was state-of-the-art yesterday might not be today. Feature sizes are getting so small that simply injecting faster or at higher temps isn’t enough. We are required to innovate and develop new processes and methods that are outside of what is considered standard injection molding. Along with the challenge of molding these micro components comes the challenge of being able to handle these components to prevent damage or not impart bio-burden. This requires robotics and custom part retrieval systems for every micro-molded component. Inspection is also an area that can be extremely challenging. Specialty metrology equipment such as micro CT scanners or laser microscopes are required, and often, customization of their normal intended use is required in order to develop a metrology process that is capable of meeting gauge R&R requirements on a tolerance that may be less the 5 microns. This expertise is accomplished through significant trial and error testing and is the enabler that allows us to succeed where others might fail. Lastly, as complicated and challenging as the micro-molding might be, the assembly of these devices can be even more complex and difficult to scale to high volume manufacturing. We are continuously having to push the limits of what is available with current vision and motion control and it is critical that we are able to design and build these systems in-house so that we control all aspects of the system. We have applications requiring 1 micron (.00004”) positional accuracy in the placement of components and this is a combination of tooling, vision, and motion precision. This can only work if you control all aspects of what is required.
Barbella: What factors must be taken into consideration in designing tooling for micromolded parts?
Shakal: A tool designer must have a clear understanding of what is possible with micro-machining in order to properly design a mold that is manufacturable. For example, it is not uncommon for us to see part features that are only a few thousandths in size. The designer must know what machining method will be optimal to put the feature into the tool steel and whether or not there are limitations with cutter reach, cutter diameter, electrode overburn, accuracy requirements, etc. Often these limitations require separating this tool steel out from the rest of the cavity and manufacturing it as a stand-alone piece. Knowing that this is required adds complexity in fitting the componentry together without giving up positional accuracy of a micron. Also, due to the extreme injection speeds and extremely small fill volumes, the molds must be able to vent properly. This is important in any mold but it is exceedingly more difficult to achieve in many micro applications. The difference from failure and success is once again at the micron level. A 1-2 micron difference in vent depth can be the difference between a part that will not fill and a part that has too much flash. It takes a pain staking attention to detail, beginning at design and carried all the way through for these applications to be a success.
Barbella: Should micromolding tooling design be outsourced? Why or why not?
Shakal: From experience, we feel very strongly that in order to provide successful results for a true micro application that is not only micro in size but also has micron level tolerances requires the supplier to be vertically integrated. Tooling is one of the key enablers to the success of a micro program and we don’t believe that we could be timely and effective with outsourced tooling. There is simply too much interaction required between the tooling and development molding teams to do this efficiently with outside resources. It is also important from our perspective that this vertical integration not only exists between tooling and molding but also part handling automation, all metrology, and automated component assembly. The tolerance stack-ups are often so tight that we must control every aspect of manufacturing. It takes considerable resources and time to build up this diverse skill set but it is critical to our customer’s success that we be able to provide this level of service.
Barbella: What new micromolding technologies (if any) are on the horizon?
Shakal: Our experience has presented us with many problems and limitations in the industry. We currently have numerous R&D efforts in process that we are planning to offer our customers in the near future. Some of these solutions include ‘gateless’ parts and the ability to fill long thin wall micro parts. We are also an early adopter of ultrasonic injection molding and are currently working with the developer of this technology on some very demanding applications where micro molding is not possible due to the limited pressure and heat that the finished product can be exposed to. This technology has also shown to have the potential of filling smaller features and longer flow lengths with significantly less pressure.