Michael Barbella, Managing Editor10.05.20
Consider, for just one moment, the ability to monitor human brain activity at its source. Imagine the knowledge that could be gleaned by directly observing the non-stop electric symphony composed and conducted by a 120 billion-piece neuronal orchestra.
Fancy gaining a ringside seat to this cerebral concerto, without the need for big, bulky machines, strange-looking skull caps, or long, tangle-prone wires. A tiny, perhaps flexible, electrode would suffice as the entrance fee.
To truly witness the magical harmony of the brain’s electric oscillations, that electrode would have to be extremely small—conceivably, 100 nanometers or so (roughly 1,000 times thinner than a human hair).
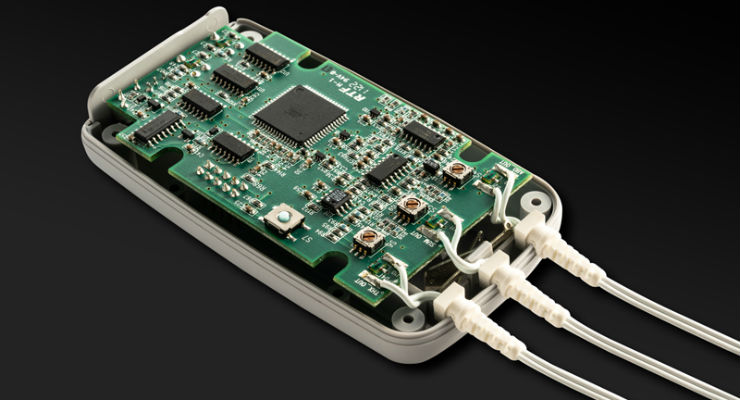
Creating an electrode of that size certainly is technologically possible. Medical electronics have steadily been shrinking over the last two decades as digital health and minimally invasive surgical procedures spawned a worldwide thirst for smaller, more complex computerized devices that improve diagnoses and tracking. The scramble for diagnostic tests, personal protective equipment, ventilators, and other medical supplies associated with the planet’s battle against COVID-19 is expected to increase demand for medical electronics over the next seven years.
Medical Product Outsourcing’s September feature, “Mission Complete,” details the various trends and challenges currently shaping the custom medical electronics market. Steve Heckman, engineer; David Gallick, senior vice president; and Joe Ogle, vice president; at P1 Technologies, were among the experts interviewed for this story. Their full input is provided in the following Q&A:
Michael Barbella: What factors must be taken into consideration when designing electronic components for medical devices?
Steve Heckman, David Gallick, Joe Ogle: For most of the products we design and manufacture at P1 Technologies we must meet U.S. and international standards for quality systems and specific product standards depending on the intended use of the end device. The number of requirements and the complexity of the requirements continue to grow year over year and present increasingly complex challenges. The medical device market has been a core focus for us for 30-plus years, and we have built and adapted our quality and regulatory systems over time to manage existing regulatory processes and adapt to new requirements as they arise.
Barbella: Please discuss some of the challenges in designing and manufacturing electronic components for medical devices. How has your company overcome these challenges?
Heckman, Gallick, Ogle: The current challenge for P1 Technologies and other companies in the medical device industry is COVID-19. Some companies are seeing significant increases in business, but many are seeing decreases in business. We have seen a downturn in some product lines, but we have been able to bring in new business to replace the losses. We are fortunate that our manufacturing is in the U.S. and most of our raw materials are sourced in the U.S., so many of the challenges that other companies with complex Asian supply chains face have not affected us. Another benefit of U.S.-based manufacturing and engineering is our ability to work closely with our customers’ engineers. Our projects are managed by engineers and they are always available to our customers through the development phases and throughout the product’s life.
Barbella: What are customers demanding or expecting in their electronic components?
Heckman, Gallick, Ogle: Customers have always wanted zero defects, low price and on-time delivery; our customers now take it for granted that they will receive these items and they are now looking for innovative custom solutions, miniaturization, and ways to get to market faster. One of the key items we offer is a comprehensive solution package that walks customers through a process from conception to product realization including regulatory requirements and validation.
Barbella: How is IoT (Internet of Things) influencing electronic component development?
Heckman, Gallick, Ogle: Being a U.S. manufacturer in a cost competitive industry, P1 Technologies has taken advantage of the Industry 4.0 technology advances to lower costs and improve quality. Advanced automation, IoT, automated communication systems and machine self-monitoring have been key items that have helped us stay cost competitive.
Barbella: How is big data influencing electronic component development?
Heckman, Gallick, Ogle: Like many manufacturers we have very good internal processes and we can consistently build a high-quality product on-time if we have good raw materials in-house. This has put a lot of emphasis on supplier selection, supplier monitoring and the cost of defects. P1 has been able to use big data from internal and external sources to better monitor suppliers and to fully understand the cost of a poor supplier and make better decisions selecting suppliers.
Barbella: How is the trend toward miniaturization of medical devices driving the design of electronic components? Please explain.
Heckman, Gallick, Ogle: A significant proportion of P1 Technology’s business is focused on interconnects and cables for wearable medical devices. This market requires miniaturization to achieve lightweight and comfortable devices that can be worn for extended periods of time. P1 also manufactures infusion cannulas and electrode systems for neuroscience research that are designed with tight tolerances and miniaturization in mind.
Barbella: In what ways is the changing regulatory environment impacting electronic component development?
Heckman, Gallick, Ogle: Growing regulatory requirements have the potential to significantly slow product development and add cost to projects. The key thing that P1 Technologies has done has been to create a flexible product development protocol in compliance with quality standards but tailored to the complexity of the project.
Barbella: The industry has grappled with a worldwide electronic components shortage in recent years. What solutions are available to tackle this problem?
Heckman, Gallick, Ogle: For the past few years we have seen component shortages in several areas and we have adapted our supply chains to overcome these problems, but for the most part these shortages have come on slowly and have been fairly limited. COVID-19 has brought about a new set of challenges that have hit us very quickly, we have seen shortages due to suppliers shutdowns, we have had delays due to shipping and we are now seeing delays due to secondary effects of COVID where COVID has significantly increased demand for one product that has taken raw materials or manufacturing resources away from parts in our supply chain.
Barbella: Component obsolescence is a challenge in the medtech industry, where the product lifecycle is very long compared to many consumer devices. How can this challenge be overcome?
Heckman, Gallick, Ogle: We try to minimize the possibility of obsolete parts during design by communicating with our suppliers and selecting raw materials that meet our end products expected life. As we progress through the product life cycle, we put contracts in place with our suppliers and stay in close contact with them so that we have as much notice as possible of parts going obsolete. There is no way to completely avoid parts going obsolete but the ability to make a last time buy and implement a change in six to 12 months is much easier than forcing a change through in two months.
Barbella: Please discuss any other trends you are noticing in electronic component development (medical devices).
Heckman, Gallick, Ogle: Most product development is driven by market needs and there is a significant amount of uncertainty in almost all markets right now. There are short-term and most likely there will be long-term disruptions in international trade due to tariffs, IP issues between the U.S. and China, and most recently COVID-19. Companies with projects in process are, for the most part, continuing development but companies are hesitant to start new development projects. In cases where there are part shortages, most companies are hesitant to buy equipment to increase capacity due to the uncertainty in long-term demand. Until there is a clearer picture on where we are going with trade barriers and when we will return to “normal” after COVID-19, it will be difficult for companies to commit to capital investment in equipment or development projects.
Fancy gaining a ringside seat to this cerebral concerto, without the need for big, bulky machines, strange-looking skull caps, or long, tangle-prone wires. A tiny, perhaps flexible, electrode would suffice as the entrance fee.
To truly witness the magical harmony of the brain’s electric oscillations, that electrode would have to be extremely small—conceivably, 100 nanometers or so (roughly 1,000 times thinner than a human hair).
Creating an electrode of that size certainly is technologically possible. Medical electronics have steadily been shrinking over the last two decades as digital health and minimally invasive surgical procedures spawned a worldwide thirst for smaller, more complex computerized devices that improve diagnoses and tracking. The scramble for diagnostic tests, personal protective equipment, ventilators, and other medical supplies associated with the planet’s battle against COVID-19 is expected to increase demand for medical electronics over the next seven years.
Medical Product Outsourcing’s September feature, “Mission Complete,” details the various trends and challenges currently shaping the custom medical electronics market. Steve Heckman, engineer; David Gallick, senior vice president; and Joe Ogle, vice president; at P1 Technologies, were among the experts interviewed for this story. Their full input is provided in the following Q&A:
Michael Barbella: What factors must be taken into consideration when designing electronic components for medical devices?
Steve Heckman, David Gallick, Joe Ogle: For most of the products we design and manufacture at P1 Technologies we must meet U.S. and international standards for quality systems and specific product standards depending on the intended use of the end device. The number of requirements and the complexity of the requirements continue to grow year over year and present increasingly complex challenges. The medical device market has been a core focus for us for 30-plus years, and we have built and adapted our quality and regulatory systems over time to manage existing regulatory processes and adapt to new requirements as they arise.
Barbella: Please discuss some of the challenges in designing and manufacturing electronic components for medical devices. How has your company overcome these challenges?
Heckman, Gallick, Ogle: The current challenge for P1 Technologies and other companies in the medical device industry is COVID-19. Some companies are seeing significant increases in business, but many are seeing decreases in business. We have seen a downturn in some product lines, but we have been able to bring in new business to replace the losses. We are fortunate that our manufacturing is in the U.S. and most of our raw materials are sourced in the U.S., so many of the challenges that other companies with complex Asian supply chains face have not affected us. Another benefit of U.S.-based manufacturing and engineering is our ability to work closely with our customers’ engineers. Our projects are managed by engineers and they are always available to our customers through the development phases and throughout the product’s life.
Barbella: What are customers demanding or expecting in their electronic components?
Heckman, Gallick, Ogle: Customers have always wanted zero defects, low price and on-time delivery; our customers now take it for granted that they will receive these items and they are now looking for innovative custom solutions, miniaturization, and ways to get to market faster. One of the key items we offer is a comprehensive solution package that walks customers through a process from conception to product realization including regulatory requirements and validation.
Barbella: How is IoT (Internet of Things) influencing electronic component development?
Heckman, Gallick, Ogle: Being a U.S. manufacturer in a cost competitive industry, P1 Technologies has taken advantage of the Industry 4.0 technology advances to lower costs and improve quality. Advanced automation, IoT, automated communication systems and machine self-monitoring have been key items that have helped us stay cost competitive.
Barbella: How is big data influencing electronic component development?
Heckman, Gallick, Ogle: Like many manufacturers we have very good internal processes and we can consistently build a high-quality product on-time if we have good raw materials in-house. This has put a lot of emphasis on supplier selection, supplier monitoring and the cost of defects. P1 has been able to use big data from internal and external sources to better monitor suppliers and to fully understand the cost of a poor supplier and make better decisions selecting suppliers.
Barbella: How is the trend toward miniaturization of medical devices driving the design of electronic components? Please explain.
Heckman, Gallick, Ogle: A significant proportion of P1 Technology’s business is focused on interconnects and cables for wearable medical devices. This market requires miniaturization to achieve lightweight and comfortable devices that can be worn for extended periods of time. P1 also manufactures infusion cannulas and electrode systems for neuroscience research that are designed with tight tolerances and miniaturization in mind.
Barbella: In what ways is the changing regulatory environment impacting electronic component development?
Heckman, Gallick, Ogle: Growing regulatory requirements have the potential to significantly slow product development and add cost to projects. The key thing that P1 Technologies has done has been to create a flexible product development protocol in compliance with quality standards but tailored to the complexity of the project.
Barbella: The industry has grappled with a worldwide electronic components shortage in recent years. What solutions are available to tackle this problem?
Heckman, Gallick, Ogle: For the past few years we have seen component shortages in several areas and we have adapted our supply chains to overcome these problems, but for the most part these shortages have come on slowly and have been fairly limited. COVID-19 has brought about a new set of challenges that have hit us very quickly, we have seen shortages due to suppliers shutdowns, we have had delays due to shipping and we are now seeing delays due to secondary effects of COVID where COVID has significantly increased demand for one product that has taken raw materials or manufacturing resources away from parts in our supply chain.
Barbella: Component obsolescence is a challenge in the medtech industry, where the product lifecycle is very long compared to many consumer devices. How can this challenge be overcome?
Heckman, Gallick, Ogle: We try to minimize the possibility of obsolete parts during design by communicating with our suppliers and selecting raw materials that meet our end products expected life. As we progress through the product life cycle, we put contracts in place with our suppliers and stay in close contact with them so that we have as much notice as possible of parts going obsolete. There is no way to completely avoid parts going obsolete but the ability to make a last time buy and implement a change in six to 12 months is much easier than forcing a change through in two months.
Barbella: Please discuss any other trends you are noticing in electronic component development (medical devices).
Heckman, Gallick, Ogle: Most product development is driven by market needs and there is a significant amount of uncertainty in almost all markets right now. There are short-term and most likely there will be long-term disruptions in international trade due to tariffs, IP issues between the U.S. and China, and most recently COVID-19. Companies with projects in process are, for the most part, continuing development but companies are hesitant to start new development projects. In cases where there are part shortages, most companies are hesitant to buy equipment to increase capacity due to the uncertainty in long-term demand. Until there is a clearer picture on where we are going with trade barriers and when we will return to “normal” after COVID-19, it will be difficult for companies to commit to capital investment in equipment or development projects.