George I’ons, Head of Product Strategy and Insights, Owen Mumford Pharmaceutical Services11.21.19
Responding to growing pressure aggravated by staff shortages, lack of funding, and an ageing population, healthcare systems in the U.S. have been instrumental in the drive towards self-administration for patients with chronic diseases. The number of Americans aged 65 and over is projected to nearly double from 52 million in 2018 to 95 million by 2060.1 By shifting the injection of certain therapies towards the home and away from the traditional clinical setting, hospitals are “outsourcing” a lower-risk medical procedure, helping to manage overcrowding within their facilities and getting patients to be more involved in their own treatment.
While this migration may be cost-effective and practical for both parties, needlestick injuries still present a significant risk within hospital settings, let alone in non-clinical sites where there is no professional presiding. In this context of growing home-administration, it is vital that equal attention be given to the safety of sharp devices and best practice injection procedures both within the confines of hospitals as well as outside. While drug delivery devices have typically been designed with professional nurses in mind, manufacturers will now need to consider the capabilities of patients when designing their products.
Typically administered via subcutaneous injection, a new wave of biological therapies for patients with chronic conditions are entering the market, which are particularly well-suited to self-administration through prefilled safety syringes. This is largely because such treatments require regular injections, for which it would be impractical and not cost effective to visit a clinic or call out a nurse each time. Patients who self-administer their own treatment are empowered by the responsibility of managing their medication regimen, thereby alleviating some of the pressure on hospitals and nurses.
While the introduction of the U.S. Needlestick Safety and Prevention Act (NSPA) almost two decades ago has led to a 30% decline in sharps injuries, they continue to occur—averaging 1,000 reported sharps injuries per day in U.S. hospitals.2 Beyond the clinical setting, it is currently estimated that over 50% of non-hospital settings are in violation of Occupational Safety and Health Administration (OSHA) laws.3 Non-compliant settings have a much higher risk of needlestick injuries occurring, leaving patients, carers, and non-users at risk of contracting more than 20 types of unwanted infections. Family members and other residential non-users are particularly vulnerable to exposure for they are often unaware of the contamination of a device or of the risk presented by a contaminated sharp. This makes them unlikely to report any contamination and get proper treatment – in fact, studies show that up to half of sharps injuries may go unreported.4 As the administration of drugs and its associated risks migrate into the homes of chronic patients, manufacturers must take on some of the responsibility of guarding non-professional users against the dangers of needlestick infections.
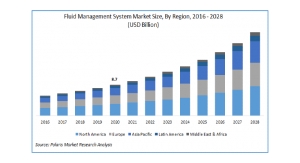
Amidst this evolving healthcare landscape, it comes as no surprise that the pre-filled safety syringe market has taken off in recent years. The design of a drug delivery device determines the level of its needlestick injury prevention capabilities, with certain designs potentially making some devices less safe than others. More specifically, devices with hollow-bore needles or syringes that retain an exposed needle after use are very high-risk.5 With reports stating that 80% of needlestick injuries can be avoided by using safer needle devices, disposable delivery devices have become instrumental in the prevention of sharps injuries and recent figures confirm this growing trend.6 The global safety syringe market is expected to reach $1.137 billion by 2023, up from $772 million in 2018—representing a CAGR growth rate of 8.1%.7
Pre-filled safety syringes are not only safer than those without needle protection but also more convenient and well-adapted to the self-administration trend. In particular, retracting needle mechanisms used in pre-filled safety syringes and auto-injectors allow both ease-of-use and safety device requirements to be met all at once, while also potentially reducing the risk of dosage errors. Allowing older, debilitated and less-dextrous patients to securely hold and operate the device without any assistance, these instruments contribute to providing patients with the independence that comes with self-administration, while reducing the risks associated with unsupervised self-care.
References:
1Population Reference Bureau (PRB), Fact Sheet: Aging in the United States, July 2019, https://www.prb.org/aging-unitedstates-fact-sheet/
2OSHA, http://www.osha.gov/SLTC/etools/hospital/hazards/sharps/sharps.html
3Medical Devices:Evidence and Research, Vol 10, Clinical, economic, and humanistic burden of needlestick injuries in healthcare workers, 2 May 2017
3https://www.cdc.gov/nora/councils/hcsa/stopsticks/sharpsinjuries.html
4World Health Organization, Needlestick Safety and Prevention, Independent Study
5World Health Organization, Needlestick Safety and Prevention, Independent Study
6Market Data Forecast, Safety Syringes Market by Technology, Oct 2018
George I’ons is head of Product Strategy and Insights at Owen Mumford Pharmaceutical Services.
While this migration may be cost-effective and practical for both parties, needlestick injuries still present a significant risk within hospital settings, let alone in non-clinical sites where there is no professional presiding. In this context of growing home-administration, it is vital that equal attention be given to the safety of sharp devices and best practice injection procedures both within the confines of hospitals as well as outside. While drug delivery devices have typically been designed with professional nurses in mind, manufacturers will now need to consider the capabilities of patients when designing their products.
Typically administered via subcutaneous injection, a new wave of biological therapies for patients with chronic conditions are entering the market, which are particularly well-suited to self-administration through prefilled safety syringes. This is largely because such treatments require regular injections, for which it would be impractical and not cost effective to visit a clinic or call out a nurse each time. Patients who self-administer their own treatment are empowered by the responsibility of managing their medication regimen, thereby alleviating some of the pressure on hospitals and nurses.
While the introduction of the U.S. Needlestick Safety and Prevention Act (NSPA) almost two decades ago has led to a 30% decline in sharps injuries, they continue to occur—averaging 1,000 reported sharps injuries per day in U.S. hospitals.2 Beyond the clinical setting, it is currently estimated that over 50% of non-hospital settings are in violation of Occupational Safety and Health Administration (OSHA) laws.3 Non-compliant settings have a much higher risk of needlestick injuries occurring, leaving patients, carers, and non-users at risk of contracting more than 20 types of unwanted infections. Family members and other residential non-users are particularly vulnerable to exposure for they are often unaware of the contamination of a device or of the risk presented by a contaminated sharp. This makes them unlikely to report any contamination and get proper treatment – in fact, studies show that up to half of sharps injuries may go unreported.4 As the administration of drugs and its associated risks migrate into the homes of chronic patients, manufacturers must take on some of the responsibility of guarding non-professional users against the dangers of needlestick infections.
Amidst this evolving healthcare landscape, it comes as no surprise that the pre-filled safety syringe market has taken off in recent years. The design of a drug delivery device determines the level of its needlestick injury prevention capabilities, with certain designs potentially making some devices less safe than others. More specifically, devices with hollow-bore needles or syringes that retain an exposed needle after use are very high-risk.5 With reports stating that 80% of needlestick injuries can be avoided by using safer needle devices, disposable delivery devices have become instrumental in the prevention of sharps injuries and recent figures confirm this growing trend.6 The global safety syringe market is expected to reach $1.137 billion by 2023, up from $772 million in 2018—representing a CAGR growth rate of 8.1%.7
Pre-filled safety syringes are not only safer than those without needle protection but also more convenient and well-adapted to the self-administration trend. In particular, retracting needle mechanisms used in pre-filled safety syringes and auto-injectors allow both ease-of-use and safety device requirements to be met all at once, while also potentially reducing the risk of dosage errors. Allowing older, debilitated and less-dextrous patients to securely hold and operate the device without any assistance, these instruments contribute to providing patients with the independence that comes with self-administration, while reducing the risks associated with unsupervised self-care.
References:
1Population Reference Bureau (PRB), Fact Sheet: Aging in the United States, July 2019, https://www.prb.org/aging-unitedstates-fact-sheet/
2OSHA, http://www.osha.gov/SLTC/etools/hospital/hazards/sharps/sharps.html
3Medical Devices:Evidence and Research, Vol 10, Clinical, economic, and humanistic burden of needlestick injuries in healthcare workers, 2 May 2017
3https://www.cdc.gov/nora/councils/hcsa/stopsticks/sharpsinjuries.html
4World Health Organization, Needlestick Safety and Prevention, Independent Study
5World Health Organization, Needlestick Safety and Prevention, Independent Study
6Market Data Forecast, Safety Syringes Market by Technology, Oct 2018
George I’ons is head of Product Strategy and Insights at Owen Mumford Pharmaceutical Services.