Sam Brusco, Associate Editor11.15.19
The drug delivery and combination products market is made up of a number of markets, all based on the means by which the drug is delivered. Some are administered through injection, some through infusion, some orally, some transdermally, and the list continues. Nanomedicine and nano delivery systems are emerging as a method of targeted drug delivery, in which particles attracted to diseases cells directly treat those cells. Implant technology can also deliver targeted medication, providing longer-lasting, localized therapy.
In order to gain more insights about the trends and challenges affecting the diverse drug delivery and combination products markets, I spoke to Bill Welch, chief technology officer of Phillips-Medisize, a Molex company. Phillips-Medisize is a Hudson, Wis.-based global outsource provider of design and manufacturing services serving the drug delivery, medical device, and primary pharmaceutical packaging markets. A portion of his input was included in the November/December issue feature article “Delivering the Goods: The Evolving World of Combination Products.” The entirety of our interview is featured here.
Sam Brusco: What were the trends that drove the biopharma and drug delivery/combination product markets in 2019?
Bill Welch: Connectivity was a highly visible topic in 2019 and will continue trending into 2020. While some biopharma companies have moved ahead with incorporating connectivity into drug delivery products, others are still formulating plans or taking a “wait and see” approach. This hesitation is generally a driven by the fear of added device cost rather than the benefits of adherence such as greater potential for improved patient outcomes and more prescriptions filled.
Another major trend behind drug delivery/combination product development is biologics. That’s because the vast majority of biologics require an injection device (including wearables). Many biologics cannot be injected in a suitable HFE span for mechanical AI, so innovations in delivery devices are required to achieve maximum efficiency and benefits to patients.
Brusco: Which latest developments and/or innovations in drug delivery technology/combination products have captured your attention?
Welch: Phillips-Medisize is already a strong player in connected medicine, which continues to be a major emphasis for us. As a market leader in drug-device combination product manufacturing, interest in our outsourcing capabilities from pharma companies continues to grow. Additionally, in cases where biopharma customers have selected a custom container requiring a dedicated filling line, we’ve seen an increased demand in collapsing the supply chain by having a single supplier such as Phillips-Medisize perform both the filling and combination product final manufacturing.
Brusco: What are the challenges and opportunities for drug delivery and combination products in the next few years?
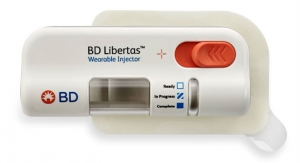
Welch: Looking ahead to the future, the nature of biologics creates a need to accommodate upwards of hundreds of thousands of combination products per year. Because production of custom devices at these high volumes is rare, there’s a real opportunity for platform device owners. The device platform evolution has resulted in many “me too” options such a mechanical AI, so there is a need for differentiation (at a competitive cost) in a crowded market. Less mature areas, such as wearable on-body injectors, present more room for innovation since that space is still developing. Finally, to produce biologics at these high volumes, more biopharma companies are looking to outsource to leaders like Phillips-Medisize, who is well positioned to handle their combination product manufacturing requirements based on our quality infrastructure and 12+ years of proven experience.
Brusco: Is there anything else you’d like to say regarding the manufacturing of drug delivery/combination products or components?
Welch: From where we sit as an established global leader who has seen the industry evolve, Phillips-Medisize helps biopharma and pharma customers around the world (U.S., Europe, and Asia) to navigate the complexities of their regulatory environment and meet the needs of patient populations with innovations that bring medicine into the digital age. We do this by closely partnering with them to design and develop custom drug delivery devices and combination products, as well as benefit from connected health solutions (including our award-winning, FDA registered Connected Health Platform) and end-to-end manufacturing services.
In order to gain more insights about the trends and challenges affecting the diverse drug delivery and combination products markets, I spoke to Bill Welch, chief technology officer of Phillips-Medisize, a Molex company. Phillips-Medisize is a Hudson, Wis.-based global outsource provider of design and manufacturing services serving the drug delivery, medical device, and primary pharmaceutical packaging markets. A portion of his input was included in the November/December issue feature article “Delivering the Goods: The Evolving World of Combination Products.” The entirety of our interview is featured here.
Sam Brusco: What were the trends that drove the biopharma and drug delivery/combination product markets in 2019?
Bill Welch: Connectivity was a highly visible topic in 2019 and will continue trending into 2020. While some biopharma companies have moved ahead with incorporating connectivity into drug delivery products, others are still formulating plans or taking a “wait and see” approach. This hesitation is generally a driven by the fear of added device cost rather than the benefits of adherence such as greater potential for improved patient outcomes and more prescriptions filled.
Another major trend behind drug delivery/combination product development is biologics. That’s because the vast majority of biologics require an injection device (including wearables). Many biologics cannot be injected in a suitable HFE span for mechanical AI, so innovations in delivery devices are required to achieve maximum efficiency and benefits to patients.
Brusco: Which latest developments and/or innovations in drug delivery technology/combination products have captured your attention?
Welch: Phillips-Medisize is already a strong player in connected medicine, which continues to be a major emphasis for us. As a market leader in drug-device combination product manufacturing, interest in our outsourcing capabilities from pharma companies continues to grow. Additionally, in cases where biopharma customers have selected a custom container requiring a dedicated filling line, we’ve seen an increased demand in collapsing the supply chain by having a single supplier such as Phillips-Medisize perform both the filling and combination product final manufacturing.
Brusco: What are the challenges and opportunities for drug delivery and combination products in the next few years?
Welch: Looking ahead to the future, the nature of biologics creates a need to accommodate upwards of hundreds of thousands of combination products per year. Because production of custom devices at these high volumes is rare, there’s a real opportunity for platform device owners. The device platform evolution has resulted in many “me too” options such a mechanical AI, so there is a need for differentiation (at a competitive cost) in a crowded market. Less mature areas, such as wearable on-body injectors, present more room for innovation since that space is still developing. Finally, to produce biologics at these high volumes, more biopharma companies are looking to outsource to leaders like Phillips-Medisize, who is well positioned to handle their combination product manufacturing requirements based on our quality infrastructure and 12+ years of proven experience.
Brusco: Is there anything else you’d like to say regarding the manufacturing of drug delivery/combination products or components?
Welch: From where we sit as an established global leader who has seen the industry evolve, Phillips-Medisize helps biopharma and pharma customers around the world (U.S., Europe, and Asia) to navigate the complexities of their regulatory environment and meet the needs of patient populations with innovations that bring medicine into the digital age. We do this by closely partnering with them to design and develop custom drug delivery devices and combination products, as well as benefit from connected health solutions (including our award-winning, FDA registered Connected Health Platform) and end-to-end manufacturing services.