Sean Fenske, Editor-in-Chief05.30.19
Given the significant medical conditions that can result from patients who suffer from peripheral artery disease, ensuring the best possible tissue oxygen monitoring and measurement techniques are used is critical. With this in mind, I was recently provided the opportunity to discuss the issue with Stephen Chad Kanick, Ph.D. Lead, Clinical Data Science, and Ben Hwang, Ph.D., Chairman and CEO of Profusa about a new alternative to more traditional monitoring/measurement options. In the following Q&A, we discussed the Lumee Oxygen Platform and what advantages it could present for patients trying to deal with peripheral artery disease.
Sean Fenske: Can you briefly explain what critical limb ischemia is?
Dr. Stephen Chad Kanick: Peripheral artery disease (PAD) is a condition that affects more than 200 million people worldwide. PAD causes the blockage of blood vessels, resulting in reduced blood flow to the lower limbs. Critical limb ischemia (CLI) is the worst form of PAD and causes either severe pain in the feet or ulcers, gangrene, or a combination of these that results in amputation if not treated. The primary problem when blood supply is choked off is decreased tissue oxygen levels in the lower limbs of CLI patients.
Fenske: Why is it important to monitor tissue oxygen in patients with this condition?
Dr. Kanick: Since PAD and CLI cause inadequate blood flow and oxygen to the lower limbs, monitoring tissue oxygen in this patient population may help to monitor disease progression, as well as guide clinical and pain management. This is important as PAD left untreated may increase the risk of wound deterioration, necrosis, sepsis, gangrene, and amputation.
Fenske: What has been the traditional method for measuring and monitoring tissue oxygen for these patients?
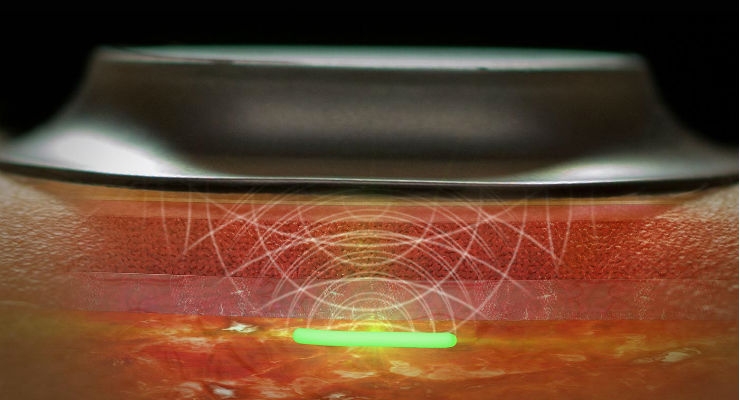
Dr. Kanick: Transcutaneous oximetry (tcpO2) is a commercially available, noninvasive technique that provides real-time information about the body’s ability to deliver oxygen to the tissue.
During tcpO2 measurement, a probe that is placed in contact with the skin surface uses a heating element to warm the underlying tissue. Additionally, an electrode is used to sample the oxygen that diffuses from the local microcirculation across the skin surface (Huch et al., 1973). The thermal challenge imposed by tcpO2 purposefully perturbs the tissue from a “baseline” physiological state to maximize local microvascular flow (Ubbink et al., 1991).
Studies have validated the tcpO2 approach in vivo by correlating transcutaneous oxygen tension (measured during heat-induced local hyperemia) with arterial oxygen supply (Huch et al., 1974; Tremper and Shoemaker, 1981). A wide range of studies have considered the use of tcpO2 to diagnose and manage patients with peripheral vascular disease or advanced critical limb ischemia (CLI) (Arsenault et al., 2012; Bacharach and Gloviczki, 1992; De Graaff et al., 2003; Hauser and Shoemaker, 1983; Štalc and Poredoš, 2002; Ubbink et al., 2000).

Components of the CE marked Lumee Oxygen Platform include the console, optical reader, custom injection pen, and controlling application.
Fenske: What is the Lumee Oxygen Platform and how does it improve upon the process of monitoring tissue oxygen?
Dr. Kanick: Profusa’s first clinical offering, the Lumee Oxygen Platform, is a tiny, injectable tissue-integrated biosensor and an intelligent data platform intended for continuous, real-time monitoring of tissue oxygen levels.
The Lumee platform may provide clinicians with a way to continuously measure tissue oxygen levels in the ischemic limb before, during, and after treatment—enabling appropriate therapy to be administered in a timely fashion before advanced and more critical symptoms appear.
A healthy volunteer feasibility study, presented at LINC, evaluated the ability of the Lumee Oxygen Platform in vivo to monitor changes in interstitial tissue oxygen in comparison with tcpO2. Results showed that the Lumee platform and tcpO2 were highly correlated, with both technologies showing a significant decrease in oxygen levels during pressure-cuff based vascular occlusion tests in the arm. Additionally, the Lumee platform detected faster rates of oxygen change during both the occlusion and recovery phases and detected reactive hyperemia in a higher percentage of tests.
Fenske: What is the status of the device with regard to market access? Where is it cleared or approved and launched?
Dr. Ben Hwang: The Lumee Oxygen Platform received a CE mark in September 2016 for the continuous monitoring of tissue oxygen. Profusa's Lumee Oxygen Platform is CE marked for sale in the EU for monitoring tissue-oxygen perfusion as a general indication.
In the U.S., the Lumee Oxygen Platform is an Investigational Device limited by Federal Law to Investigational Use.
Fenske: What were the primary challenges with the development of this technology and how did you overcome it?
Dr. Ben Hwang: With Profusa’s unique hydrogel technology, the company is attempting to overcome or mitigate foreign-body reactions that are often related to big and rigid implants or inserts, and the trauma created in placing such devices. We developed our sensors for 10 years to show that it integrates in the body in a different way due to its tissue-integrating properties.
Each tiny biosensor is a soft, flexible fiber designed to be biologically compatible with the body’s tissues (made of a porous “smart gel”). The biosensor provides real-time, in-body monitoring of biochemical data while overcoming the effects of local inflammation or rejection by the body.
Fenske: What are the next steps for this technology?
Dr. Ben Hwang: Following its August 2018 $45 million Series C financing round, Profusa continues to focus on advancing the commercialization of its Lumee platform (pushing forward on FDA approval), as well as the development of its transformative glucose biosensor technology.
Earlier this year at the FLEX 2019 conference, Profusa and NextFlex unveiled a skin-worn reader prototype for continuous oxygen monitoring that works in conjunction with the Lumee Oxygen Platform.
The company is also backed by and in partnership with the Defense Advanced Research Projects Agency, National Institute of Health, as well as leading research universities such as North Carolina State University’s ASSIST Center to further develop sensors that monitor patients with PAD and provide real-time monitoring of a combat soldier’s health status.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Dr. Ben Hwang: We’re pleased the data presented at LINC helps to build our foundation of continuous research with the Lumee platform.
As Dr. Peter Schneider, vascular surgeon and clinical researcher in limb salvage vascular procedures at Kaiser Permanente from Honolulu, Hawaii, who presented findings from the healthy volunteer feasibility study stated, “Measurements of regional tissue oxygen serve as a proxy to monitor local perfusion and have the potential to guide crucial therapeutic decisions in multiple clinical disciplines for peripheral artery disease, and wound management. These decisions are now made almost completely on the basis of clinical judgement alone without guidance. These findings show promise that the Lumee Oxygen Platform can become a valuable tool for clinicians when they need to assess perfusion."
Research on measuring changes in tissue oxygen with the Lumee Oxygen Platform was also published on Microvascular Research.
Sean Fenske: Can you briefly explain what critical limb ischemia is?
Dr. Stephen Chad Kanick: Peripheral artery disease (PAD) is a condition that affects more than 200 million people worldwide. PAD causes the blockage of blood vessels, resulting in reduced blood flow to the lower limbs. Critical limb ischemia (CLI) is the worst form of PAD and causes either severe pain in the feet or ulcers, gangrene, or a combination of these that results in amputation if not treated. The primary problem when blood supply is choked off is decreased tissue oxygen levels in the lower limbs of CLI patients.
Fenske: Why is it important to monitor tissue oxygen in patients with this condition?
Dr. Kanick: Since PAD and CLI cause inadequate blood flow and oxygen to the lower limbs, monitoring tissue oxygen in this patient population may help to monitor disease progression, as well as guide clinical and pain management. This is important as PAD left untreated may increase the risk of wound deterioration, necrosis, sepsis, gangrene, and amputation.
Fenske: What has been the traditional method for measuring and monitoring tissue oxygen for these patients?
Dr. Kanick: Transcutaneous oximetry (tcpO2) is a commercially available, noninvasive technique that provides real-time information about the body’s ability to deliver oxygen to the tissue.
During tcpO2 measurement, a probe that is placed in contact with the skin surface uses a heating element to warm the underlying tissue. Additionally, an electrode is used to sample the oxygen that diffuses from the local microcirculation across the skin surface (Huch et al., 1973). The thermal challenge imposed by tcpO2 purposefully perturbs the tissue from a “baseline” physiological state to maximize local microvascular flow (Ubbink et al., 1991).
Studies have validated the tcpO2 approach in vivo by correlating transcutaneous oxygen tension (measured during heat-induced local hyperemia) with arterial oxygen supply (Huch et al., 1974; Tremper and Shoemaker, 1981). A wide range of studies have considered the use of tcpO2 to diagnose and manage patients with peripheral vascular disease or advanced critical limb ischemia (CLI) (Arsenault et al., 2012; Bacharach and Gloviczki, 1992; De Graaff et al., 2003; Hauser and Shoemaker, 1983; Štalc and Poredoš, 2002; Ubbink et al., 2000).

Components of the CE marked Lumee Oxygen Platform include the console, optical reader, custom injection pen, and controlling application.
Dr. Kanick: Profusa’s first clinical offering, the Lumee Oxygen Platform, is a tiny, injectable tissue-integrated biosensor and an intelligent data platform intended for continuous, real-time monitoring of tissue oxygen levels.
The Lumee platform may provide clinicians with a way to continuously measure tissue oxygen levels in the ischemic limb before, during, and after treatment—enabling appropriate therapy to be administered in a timely fashion before advanced and more critical symptoms appear.
A healthy volunteer feasibility study, presented at LINC, evaluated the ability of the Lumee Oxygen Platform in vivo to monitor changes in interstitial tissue oxygen in comparison with tcpO2. Results showed that the Lumee platform and tcpO2 were highly correlated, with both technologies showing a significant decrease in oxygen levels during pressure-cuff based vascular occlusion tests in the arm. Additionally, the Lumee platform detected faster rates of oxygen change during both the occlusion and recovery phases and detected reactive hyperemia in a higher percentage of tests.
Fenske: What is the status of the device with regard to market access? Where is it cleared or approved and launched?
Dr. Ben Hwang: The Lumee Oxygen Platform received a CE mark in September 2016 for the continuous monitoring of tissue oxygen. Profusa's Lumee Oxygen Platform is CE marked for sale in the EU for monitoring tissue-oxygen perfusion as a general indication.
In the U.S., the Lumee Oxygen Platform is an Investigational Device limited by Federal Law to Investigational Use.
Fenske: What were the primary challenges with the development of this technology and how did you overcome it?
Dr. Ben Hwang: With Profusa’s unique hydrogel technology, the company is attempting to overcome or mitigate foreign-body reactions that are often related to big and rigid implants or inserts, and the trauma created in placing such devices. We developed our sensors for 10 years to show that it integrates in the body in a different way due to its tissue-integrating properties.
Each tiny biosensor is a soft, flexible fiber designed to be biologically compatible with the body’s tissues (made of a porous “smart gel”). The biosensor provides real-time, in-body monitoring of biochemical data while overcoming the effects of local inflammation or rejection by the body.
Fenske: What are the next steps for this technology?
Dr. Ben Hwang: Following its August 2018 $45 million Series C financing round, Profusa continues to focus on advancing the commercialization of its Lumee platform (pushing forward on FDA approval), as well as the development of its transformative glucose biosensor technology.
Earlier this year at the FLEX 2019 conference, Profusa and NextFlex unveiled a skin-worn reader prototype for continuous oxygen monitoring that works in conjunction with the Lumee Oxygen Platform.
The company is also backed by and in partnership with the Defense Advanced Research Projects Agency, National Institute of Health, as well as leading research universities such as North Carolina State University’s ASSIST Center to further develop sensors that monitor patients with PAD and provide real-time monitoring of a combat soldier’s health status.
Fenske: Do you have any additional comments or thoughts you’d like to share?
Dr. Ben Hwang: We’re pleased the data presented at LINC helps to build our foundation of continuous research with the Lumee platform.
As Dr. Peter Schneider, vascular surgeon and clinical researcher in limb salvage vascular procedures at Kaiser Permanente from Honolulu, Hawaii, who presented findings from the healthy volunteer feasibility study stated, “Measurements of regional tissue oxygen serve as a proxy to monitor local perfusion and have the potential to guide crucial therapeutic decisions in multiple clinical disciplines for peripheral artery disease, and wound management. These decisions are now made almost completely on the basis of clinical judgement alone without guidance. These findings show promise that the Lumee Oxygen Platform can become a valuable tool for clinicians when they need to assess perfusion."
Research on measuring changes in tissue oxygen with the Lumee Oxygen Platform was also published on Microvascular Research.