William Stearns, Associate Senior Engineer, Intertek08.13.18
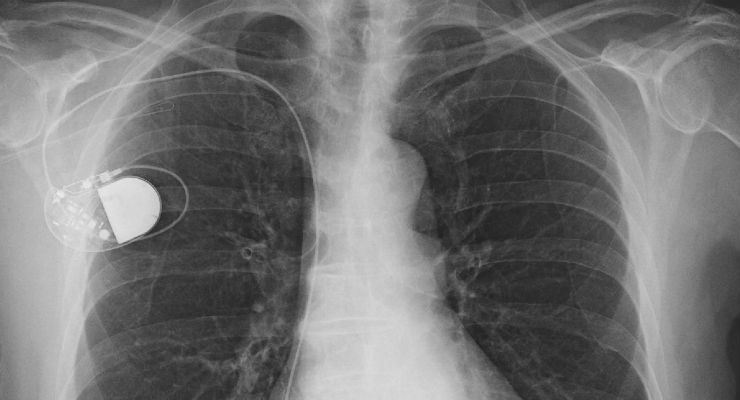
Active implantable medical devices (AIMDs) rely on a source of power other than that provided by the body or gravity, and are intended to be totally or partially introduced into the patient and remain in place. There is a wide range of AIMDs: pacemakers, defibrillators, infusion pumps, cochlear implants, and neurostimulators.
Most AIMDs consist of both the product inserted into the body and non-implantable supporting equipment, both of which require testing and evaluation to medical regulatory standards. It is important for manufacturers to be familiar with both aspects and the requirements.
The implantable device should be evaluated to the ISO 14708 or EN 45502 family of standards, “Implants for surgery -- Active implantable medical devices,” which has seven parts. Devices will not necessarily need to comply with the entirety of this standard family—it will depend on the product type. Here is an overview of the different sections:
The requirements of Parts 2-7 listed above supplement or modify those of Part 1. The requirements of each subsequent part take priority over those of Part 1, as they indicate requirements, procedures, and methods of illustrating compliance specific to their product type. In addition, each section includes information applicable to non-implantable parts and accessories of the device.
Under Part 1 of ISO 14708 for AIMDs, non-implantable supporting equipment must be evaluated to the specifications to certain electrical standards, including:
Additionally, many new AIMD systems communicate wirelessly with the implant and other external equipment. These interfaces require additional testing to cover the wireless technology and communication. With so many factors to consider, it’s important to begin with a clear evaluation strategy to ensure your device meets the necessary requirements.
Evaluating the implantable and non-implantable equipment simultaneously has several advantages, including savings in time and costs, so manufacturers should consider an approach that incorporates both testing types. Simultaneous testing allows for economies of scale, as both testing types have requirements around risk management, software, usability, and references to each other. This will allow for references between reports without having to duplicate efforts, and ultimately a single report package that contains all necessary information for the FDA or notified body. The body can then properly review both the implantable device and the supporting external equipment for a potentially faster release into the market.
A design review early in the design cycle to go over the product and process can prevent costly mistakes. Make sure your team reviews the product, considering its intended use, user, environment, and evaluations requirements. Many issues are quite common and can be resolved easily when it is early in the design. Combatting problems early in the design phase can save costly redesigns and additional testing later, and can allow you to avoid delays to market.
AIMDs are complex devices subject to rigorous regulatory standards by authorities across the globe. With both implantable and non-implantable factors to consider, this means manufacturers have a lengthy list of standards to know and comply with. Keeping them in mind from the beginning design phase and completing evaluations in a timely, cost-effective manner can help bring these devices to markets across the globe. Make sure you know which standards apply to your product, know them well, and prepare yourself for the testing you’ll need to complete.
William Stearns is an associate senior engineer at Intertek, a London, U.K.-based total quality assurance provider to industries worldwide.
Most AIMDs consist of both the product inserted into the body and non-implantable supporting equipment, both of which require testing and evaluation to medical regulatory standards. It is important for manufacturers to be familiar with both aspects and the requirements.
The implantable device should be evaluated to the ISO 14708 or EN 45502 family of standards, “Implants for surgery -- Active implantable medical devices,” which has seven parts. Devices will not necessarily need to comply with the entirety of this standard family—it will depend on the product type. Here is an overview of the different sections:
- Part 1: This standard specifies general requirements for AIMDs to provide basic assurance of safety for patients and users. It also includes obligations for product marking and information/documentation to provide patients/users to minimize the chance of product misuse.
- Part 2: This portion of the standard covers specific requirements for cardiac pacemakers.
- Part 3: This section covers the requirements for implantable neurostimulators, to provide basic assurance of safety for both patients and users. It covers devices intended for electrical stimulation of the central or peripheral nervous system. Part 3 aligns with ISO 14708-1:2014/EN 45502:2015
- Part 4: Specific requirements for implantable infusion pumps, devices intended to deliver medicinal substances to site-specific locations within the human body. It covers safety requirements that are illustrated via type testing of samples.
- Part 5: This section applies to circulatory support devices, excluding intra-aortic balloon pumps, external corporeal perfusion devices, and cardiomyoplasty. The standard specifies type tests, animal studies, and clinical evaluation requirements required to show compliance with this standard.
- Part 6: This section addresses specific requirements for AIMDs intended to treat tachyarrhythmia, including implantable defibrillators.
- Part 7: This standard specifies requirements applicable to AIMDs intended to treat hearing impairment via electrical stimulation of the auditory pathways, also known as cochlear implant systems. It includes type test specifications for these products.
The requirements of Parts 2-7 listed above supplement or modify those of Part 1. The requirements of each subsequent part take priority over those of Part 1, as they indicate requirements, procedures, and methods of illustrating compliance specific to their product type. In addition, each section includes information applicable to non-implantable parts and accessories of the device.
Under Part 1 of ISO 14708 for AIMDs, non-implantable supporting equipment must be evaluated to the specifications to certain electrical standards, including:
- IEC 60601‑1:2005 Part 1.This standard covers general requirements for basic safety and essential performance of medical electrical equipment.
- IEC 60601-1-6. General requirements for basic safety and essential performance, collateral standard for Usability. IEC 60601-1-6 requires compliance to IEC 62366, which covers the application of usability engineering to medical devices.
- IEC 60601-1-2. This standard is specific for electromagnetic compatibility (EMC) of medical electrical devices to ensure their safety and performance in proximity to other electrical products.
- IEC 62304. A standard that specifies life cycle requirements for the development of medical software and software used within medical devices, it includes provisions for risk management, maintenance, and configuration.
- IEC 60601-1-11 Part 1-11. This collateral standard covers requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment and should be used for any device intended for home use.
Additionally, many new AIMD systems communicate wirelessly with the implant and other external equipment. These interfaces require additional testing to cover the wireless technology and communication. With so many factors to consider, it’s important to begin with a clear evaluation strategy to ensure your device meets the necessary requirements.
Evaluating the implantable and non-implantable equipment simultaneously has several advantages, including savings in time and costs, so manufacturers should consider an approach that incorporates both testing types. Simultaneous testing allows for economies of scale, as both testing types have requirements around risk management, software, usability, and references to each other. This will allow for references between reports without having to duplicate efforts, and ultimately a single report package that contains all necessary information for the FDA or notified body. The body can then properly review both the implantable device and the supporting external equipment for a potentially faster release into the market.
A design review early in the design cycle to go over the product and process can prevent costly mistakes. Make sure your team reviews the product, considering its intended use, user, environment, and evaluations requirements. Many issues are quite common and can be resolved easily when it is early in the design. Combatting problems early in the design phase can save costly redesigns and additional testing later, and can allow you to avoid delays to market.
AIMDs are complex devices subject to rigorous regulatory standards by authorities across the globe. With both implantable and non-implantable factors to consider, this means manufacturers have a lengthy list of standards to know and comply with. Keeping them in mind from the beginning design phase and completing evaluations in a timely, cost-effective manner can help bring these devices to markets across the globe. Make sure you know which standards apply to your product, know them well, and prepare yourself for the testing you’ll need to complete.
William Stearns is an associate senior engineer at Intertek, a London, U.K.-based total quality assurance provider to industries worldwide.