Caroline Lee, Account Representative, Mintz & Hoke04.11.18
The term “never event” was coined by Ken Kizer, M.D., a former CEO of the National Quality Forum, to address shocking medical errors that should never happen. One such tragic event caused the death of a patient who received nutrition through a medical device intended for respiratory purposes. While not all outcomes from tubing misconnections are never events, negative outcomes can occur across a vast expanse of medical practices that require medical devices and connectors, including breathing and respiratory systems, interventional radiology and endoscopy devices, as well as many devices for general surgery, to name a few.
Recognizing the same luer connector was in use for several different purposes, an industry alliance was formed to help introduce international standards in medical device tubing connectors that enhance patient safety. The alliance, known as the Global Enteral Device Supplier Association (GEDSA), was charged with coordinating the design, development, and marketing launch of a new standard.
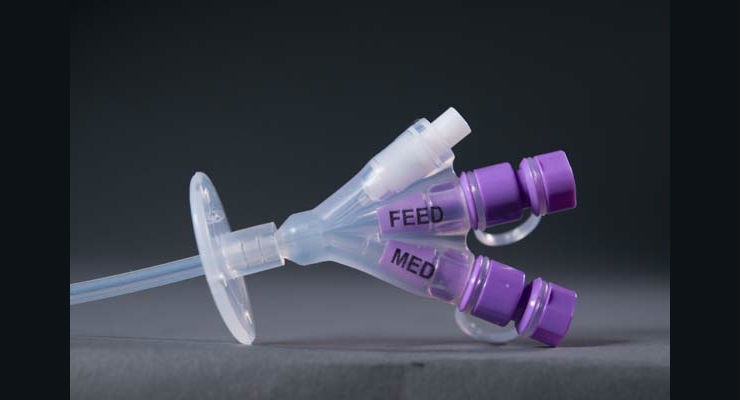
The resulting standard, ISO 80369-3, spurred the innovation of a new connector under the trade name ENFit. This particular connector is specific to enteral feeding and ensures only those devices intended for nutritional delivery connect with the corresponding tubing. Completely reengineering some of the most commonly used medical devices required a manufacturer that was up for a challenge.
A Spectrum Plastics Group company formerly known in the market as Xeridiem Medical Devices took an early lead by investing in the engineering, regulatory compliance, and manufacture of these devices. They are available in a private label, turnkey product and as a legal manufacturer with 510(k) clearance, Health Canada registrations and CE marks. Spectrum Plastics Group has also made this available to interested global medical device OEMs for a fraction of what any one company would have spent on such a sustaining engineering program.
“We are a charter member, among the very first, who joined GEDSA and their commitment to encouraging hospitals and clinics to make this change,” said Paul Melnychuck, senior director of business development and innovation at Spectrum Plastics Group. Ultimately, there will likely be six groups of connector products to address different medical disciplines. The new product on the horizon, NRFit, is a neuraxial connector for epidural purposes. Completing the family of ISO 80369 standards for these small bore connectors include other medical applications such as breathing systems and driving gases applications, limb cuff inflation applications, and connectors with 6 percent (luer) taper for intravascular or hypodermic applications. Connectors for urethral and urinary applications are planned. ENFit connectors are already widely adopted across Europe and are essentially required by law in the state of California; other states and global regions may follow suit. Complete ENFit replacement of legacy luer-based devices will continue to take at least two more years, but the industry is at about 25 percent adoption today, projected to reach about 67 percent by the end of 2018, and could reach 100 percent by the year 2020.
Melnychuck continued, “Our ENFit capable products include a connector/cap/tether combination that also features a ‘winged barb’ design to the proximal end of the device. By using this more robust, mechanical (and not chemical-bonded) approach, we achieve a higher level of product consistency than achieved through a more conventional bonded design.”
“The highlight of this story is that Spectrum Plastics Group had the foresight, commitment, and expertise to invest in the solution,” said Melnychuck. “We anticipated the market need for ENFit and absorbed the risk in the engineering and product development of this new device platform. Our OEM partners value our ENFit capable solutions, and choose to build a catalog of distributed finished devices around it, because we can provide it at a fraction of the cost versus investing their own time and resources into engineering the same product—especially if enteral feeding is tangential to their core business in another medical intervention. As an ISO standard, there is not much product differentiation and value to be gained by reinventing the wheel and wholly shouldering the cost.”
Recognizing the same luer connector was in use for several different purposes, an industry alliance was formed to help introduce international standards in medical device tubing connectors that enhance patient safety. The alliance, known as the Global Enteral Device Supplier Association (GEDSA), was charged with coordinating the design, development, and marketing launch of a new standard.
The resulting standard, ISO 80369-3, spurred the innovation of a new connector under the trade name ENFit. This particular connector is specific to enteral feeding and ensures only those devices intended for nutritional delivery connect with the corresponding tubing. Completely reengineering some of the most commonly used medical devices required a manufacturer that was up for a challenge.
A Spectrum Plastics Group company formerly known in the market as Xeridiem Medical Devices took an early lead by investing in the engineering, regulatory compliance, and manufacture of these devices. They are available in a private label, turnkey product and as a legal manufacturer with 510(k) clearance, Health Canada registrations and CE marks. Spectrum Plastics Group has also made this available to interested global medical device OEMs for a fraction of what any one company would have spent on such a sustaining engineering program.
“We are a charter member, among the very first, who joined GEDSA and their commitment to encouraging hospitals and clinics to make this change,” said Paul Melnychuck, senior director of business development and innovation at Spectrum Plastics Group. Ultimately, there will likely be six groups of connector products to address different medical disciplines. The new product on the horizon, NRFit, is a neuraxial connector for epidural purposes. Completing the family of ISO 80369 standards for these small bore connectors include other medical applications such as breathing systems and driving gases applications, limb cuff inflation applications, and connectors with 6 percent (luer) taper for intravascular or hypodermic applications. Connectors for urethral and urinary applications are planned. ENFit connectors are already widely adopted across Europe and are essentially required by law in the state of California; other states and global regions may follow suit. Complete ENFit replacement of legacy luer-based devices will continue to take at least two more years, but the industry is at about 25 percent adoption today, projected to reach about 67 percent by the end of 2018, and could reach 100 percent by the year 2020.
Melnychuck continued, “Our ENFit capable products include a connector/cap/tether combination that also features a ‘winged barb’ design to the proximal end of the device. By using this more robust, mechanical (and not chemical-bonded) approach, we achieve a higher level of product consistency than achieved through a more conventional bonded design.”
“The highlight of this story is that Spectrum Plastics Group had the foresight, commitment, and expertise to invest in the solution,” said Melnychuck. “We anticipated the market need for ENFit and absorbed the risk in the engineering and product development of this new device platform. Our OEM partners value our ENFit capable solutions, and choose to build a catalog of distributed finished devices around it, because we can provide it at a fraction of the cost versus investing their own time and resources into engineering the same product—especially if enteral feeding is tangential to their core business in another medical intervention. As an ISO standard, there is not much product differentiation and value to be gained by reinventing the wheel and wholly shouldering the cost.”