Kate Reifers, Co-Production International03.16.18
Expanding medical device and orthopedic manufacturing operations outside of the U.S. often carries the misconception that it’s about saving a failing business. When it comes to expanding to Mexico, nothing could be further from the truth.
Major medical device manufacturers utilize three major methods for expanding operations into Mexico: using a shelter company provider, setting up an independent corporation, and/or working with contract manufacturers. The long-standing ties between the United States and Mexico have fostered a solid and binational pro-business environment. Preferential tariffs and North American trade deals have created an efficient infrastructure for moving goods throughout the continent with zero or nominal tariffs, while our respective governments have streamlined regulations allowing businesses to set up operations with minimal obstacles.
Before we jump into the methods for getting started in Mexico, let’s take a look at its robust medical device industry.
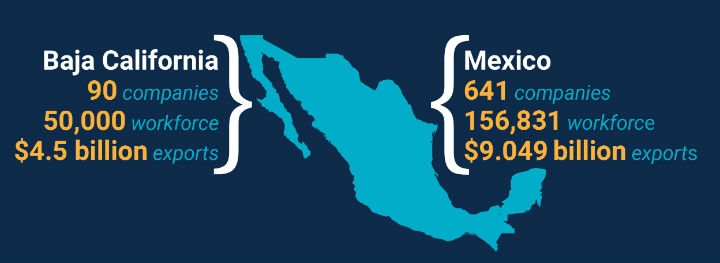
In the last few decades, over 641 medical device manufacturers have landed in nearshore Mexico and are now responsible for over $9.049 billion in annual exports (ProMexico, 2018). In this same time frame, U.S. and international manufacturers in Mexico are nowthe largest exporters of medical devices to the U.S. and Latin America, and the 8th largest exporters of medical devices in the world.
Why Did Mexico Become the Destination for Manufacturers?
Cost-effective opportunities for growth and proximity to U.S. operations and North American markets are the main drivers for medical device and orthopedic manufacturers.
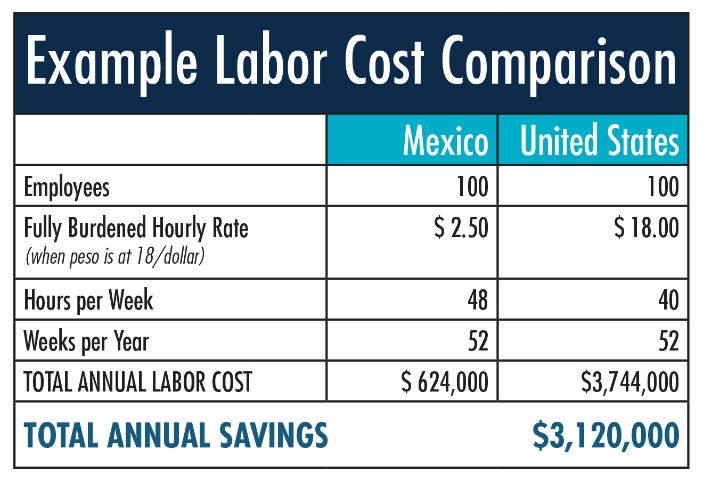
Mexico’s medical device workforce is highly-trained, less expensive, and plentiful with over 156,000 dedicated workers in this sector alone. With strong academic-government-private sector collaboration, every day Mexican medical device workers are making pacemakers, orthopedic devices, surgical fixtures, intravenous bands, imaging machines and more—most destined for U.S. markets. These labor cost savings alone represent a huge draw for manufacturers wishing to maintain the same quality as their U.S. facilities, while freeing up capital to invest in innovation and expand their global footprint.
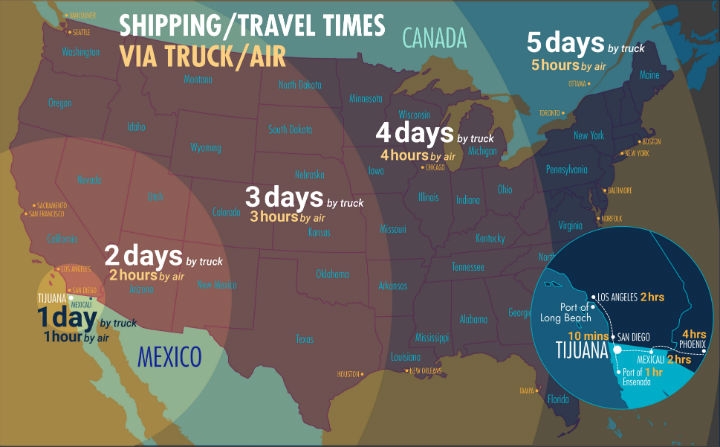
Proximity represents several cost-effective benefits over other global locations—operational oversight and just-in-time logistics and transportation to North American markets. Month-long or more shipping time frames from overseas just don’t cut it anymore. Additionally, within a few hours by plane or car, U.S. executives and their personnel can be on-site for reviews, training, and production oversight. As with many manufacturing sectors, U.S. manufacturers are also using their Mexican facilities as co-production opportunities where their U.S. and Mexican staff communicate and train together regularly.
“We found that buildings were readily available, and business and labor costs were better than many Latin American countries. And you can’t beat the physical proximity. We [also] ruled out China because it was not a good fit. Beyond the political situation and rising labor and shipping costs, it was too far away for us to manage,” said Ben Prime, technical manager for Phase 2, a contract manufacturer of single-use, disposable medical devices. In 2015, Phase 2 expanded into Tijuana with new 30,000 square foot facility that is FDA registered and certified to ISO 13485 with ISO Class 8 clean rooms.
Tijuana: North America’s Largest Medical Manufacturing Hub
Mexican border cities such as Tijuana, located just 20 minutes south of San Diego, Calif., have invested heavily in manufacturing industry infrastructure. Approximately 70 percent of the world’s largest medical device and orthopedic manufacturers have operations in Tijuana, including the major mainstays CareFusion, Greatbatch Medical, DJO Global, Medtronics, Smiths, and Cardinal Health.
Now the largest medical device manufacturing hub in North America, Tijuana offers opportunities like a frontier town, but with the physical, governmental, and utilities infrastructure of a modern city keen on keeping business moving. Well-maintained highways, significant investments in commercial ports of entry by both U.S. and Mexican customs, and governmental and private sector support staff are all aimed at creating a cost-effective and efficient environment for manufacturers to thrive and grow.
Facilities in Tijuana make FDA Class I, II, and III products in ISO certified plants, and in Class 100 to 100,000 clean rooms. Companies like Brentwood Industries, who specialize in medical thermoforming and plastic injection molding, chose Tijuana for operational redundancy and proximity to existing customers. Brentwood leased a 35,000 square foot facility in Tijuana at the end of 2017 and will begin production later this quarter.
“Tijuana’s continued development as a major medical manufacturing hub has placed it on our radar since 2014,” said Walter Banta, senior director of market development for Brentwood Industries. “Brentwood has many existing medical customers in Tijuana. By opening a manufacturing site in close proximity to our existing customer base, we can better serve them by offering faster order turnaround times, reduced freight costs, and strategic inventory control options.”
Three Methods Global Medical Device Manufacturers Used to Get Started in Mexico
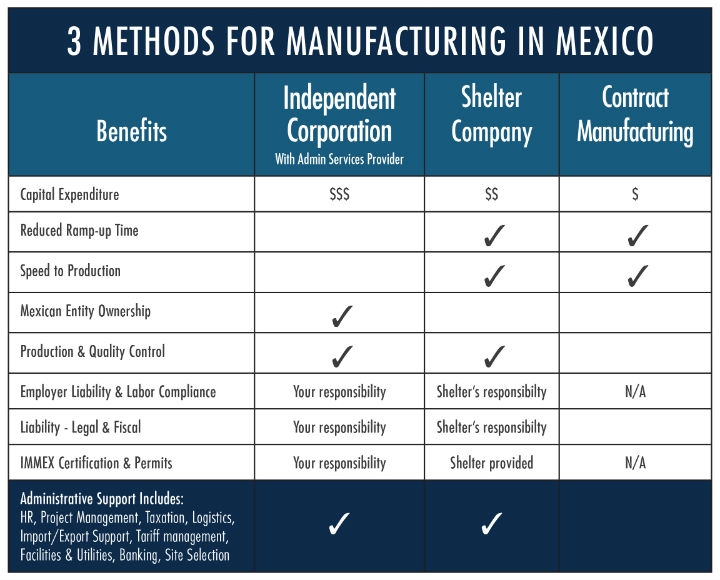
Some manufacturers have dedicated expansion teams for foreign site selection and management that allow them to start up on their own. Even so, the cost, learning curve, and slower ramp-up times of going it alone necessitate local experts to get up and running within desired timelines. That said, there are three major approaches for expanding into Mexico:
The Shelter Company Method
Using a shelter company provider is the most popular of these methods. Think of the Mexican shelter company acting as an already existing umbrella that allows your company to come in out of the rain without having to build the umbrella from scratch yourself. In not so many words, the shelter company method is faster, safer, and the most cost-effective way to land in Mexico.
By operating under a shelter, your company takes advantage of the shelter’s already existing permits, certifications, legal structure, and local administrative support staff. Not only are the liabilities and risks limited when under a shelter company, but the annual cost to operate is significantly lower than running an operation as a standalone independent corporation. The entire responsibility for your operation falls on the shelter company provider, from facility permitting, legal and fiscal obligations, to labor law and environmental law compliance. Using a shelter company provider can save newly-landed companies anywhere from 12–25 percent in annual operational costs for facilities with 50-100 employees.
“We considered a number of options, including the possibility of opening our own facility or utilizing a shelter service. We considered locations such as Mexicali, Ciudad Juarez, Rosarito, and Tijuana. We chose Tijuana for its proximity to our Irvine, California Headquarters, the quality of the workforce in Tijuana and the ability to work with a shelter company provider,” said John Hamilton, Aspen Medical Products’ vice president of strategic initiatives and special projects. The California-based Aspen Medical Products is a leader in spinal bracing products and orthotics.
Working with a shelter company provider allows the manufacturer to focus 100 percent of their efforts on the manufacturing side while the provider handles all the background details. The manufacturer is still involved in major decisions, especially in regard to site selection, build out, and recruiting qualifications. Companies that utilize a shelter company provider can stay under the shelter exactly as long as they want to. Some choose to permanently remain under the shelter and others, over time, transition to their own independent corporation while still utilizing the local administrative support offered by the provider.
The Independent Mexican Corporation
Starting off with an independent corporation is the second most popular method. Establishing your independent corporation means setting up your own Mexican corporate entity and obtaining your own permits, such as the IMMEX certification for tariff-free temporary imports. Firms that offer shelter company services will usually offer this option as well, allowing manufacturers to establish their own business entity while providing support along the way.
The ramp-up time is longer and the exposure greater, though often a choice for manufacturers with 500 employees or more and those wishing to establish a long-term presence in the region. Additionally, some OEMs just prefer to have their own Mexican corporation. Manufacturers who choose the independent corporation option will often work with local shelter company firms, taking advantage of their team’s administrative, legal, HR, and tax expertise familiar with both U.S. and Mexican laws.
The medical device manufacturing industry in Mexico is not a passing fad, but rather a robust, well-established and well-supported mega-region. With the global medical device market expected to reach $532.2 billion by 2024, will your company join the ranks of top medical device manufacturers in Mexico?
Kate Reifers is a writer and researcher for Co-Production International, specializing in manufacturing in Mexico and Baja California.
Major medical device manufacturers utilize three major methods for expanding operations into Mexico: using a shelter company provider, setting up an independent corporation, and/or working with contract manufacturers. The long-standing ties between the United States and Mexico have fostered a solid and binational pro-business environment. Preferential tariffs and North American trade deals have created an efficient infrastructure for moving goods throughout the continent with zero or nominal tariffs, while our respective governments have streamlined regulations allowing businesses to set up operations with minimal obstacles.
Before we jump into the methods for getting started in Mexico, let’s take a look at its robust medical device industry.

In the last few decades, over 641 medical device manufacturers have landed in nearshore Mexico and are now responsible for over $9.049 billion in annual exports (ProMexico, 2018). In this same time frame, U.S. and international manufacturers in Mexico are nowthe largest exporters of medical devices to the U.S. and Latin America, and the 8th largest exporters of medical devices in the world.
Why Did Mexico Become the Destination for Manufacturers?
Cost-effective opportunities for growth and proximity to U.S. operations and North American markets are the main drivers for medical device and orthopedic manufacturers.

Mexico’s medical device workforce is highly-trained, less expensive, and plentiful with over 156,000 dedicated workers in this sector alone. With strong academic-government-private sector collaboration, every day Mexican medical device workers are making pacemakers, orthopedic devices, surgical fixtures, intravenous bands, imaging machines and more—most destined for U.S. markets. These labor cost savings alone represent a huge draw for manufacturers wishing to maintain the same quality as their U.S. facilities, while freeing up capital to invest in innovation and expand their global footprint.

Proximity represents several cost-effective benefits over other global locations—operational oversight and just-in-time logistics and transportation to North American markets. Month-long or more shipping time frames from overseas just don’t cut it anymore. Additionally, within a few hours by plane or car, U.S. executives and their personnel can be on-site for reviews, training, and production oversight. As with many manufacturing sectors, U.S. manufacturers are also using their Mexican facilities as co-production opportunities where their U.S. and Mexican staff communicate and train together regularly.
“We found that buildings were readily available, and business and labor costs were better than many Latin American countries. And you can’t beat the physical proximity. We [also] ruled out China because it was not a good fit. Beyond the political situation and rising labor and shipping costs, it was too far away for us to manage,” said Ben Prime, technical manager for Phase 2, a contract manufacturer of single-use, disposable medical devices. In 2015, Phase 2 expanded into Tijuana with new 30,000 square foot facility that is FDA registered and certified to ISO 13485 with ISO Class 8 clean rooms.
Tijuana: North America’s Largest Medical Manufacturing Hub
Mexican border cities such as Tijuana, located just 20 minutes south of San Diego, Calif., have invested heavily in manufacturing industry infrastructure. Approximately 70 percent of the world’s largest medical device and orthopedic manufacturers have operations in Tijuana, including the major mainstays CareFusion, Greatbatch Medical, DJO Global, Medtronics, Smiths, and Cardinal Health.
Now the largest medical device manufacturing hub in North America, Tijuana offers opportunities like a frontier town, but with the physical, governmental, and utilities infrastructure of a modern city keen on keeping business moving. Well-maintained highways, significant investments in commercial ports of entry by both U.S. and Mexican customs, and governmental and private sector support staff are all aimed at creating a cost-effective and efficient environment for manufacturers to thrive and grow.
Facilities in Tijuana make FDA Class I, II, and III products in ISO certified plants, and in Class 100 to 100,000 clean rooms. Companies like Brentwood Industries, who specialize in medical thermoforming and plastic injection molding, chose Tijuana for operational redundancy and proximity to existing customers. Brentwood leased a 35,000 square foot facility in Tijuana at the end of 2017 and will begin production later this quarter.
“Tijuana’s continued development as a major medical manufacturing hub has placed it on our radar since 2014,” said Walter Banta, senior director of market development for Brentwood Industries. “Brentwood has many existing medical customers in Tijuana. By opening a manufacturing site in close proximity to our existing customer base, we can better serve them by offering faster order turnaround times, reduced freight costs, and strategic inventory control options.”
Three Methods Global Medical Device Manufacturers Used to Get Started in Mexico
Some manufacturers have dedicated expansion teams for foreign site selection and management that allow them to start up on their own. Even so, the cost, learning curve, and slower ramp-up times of going it alone necessitate local experts to get up and running within desired timelines. That said, there are three major approaches for expanding into Mexico:
- Shelter Company
- Independent Company with Local Admin Support
- Contract Manufacturing

The Shelter Company Method
Using a shelter company provider is the most popular of these methods. Think of the Mexican shelter company acting as an already existing umbrella that allows your company to come in out of the rain without having to build the umbrella from scratch yourself. In not so many words, the shelter company method is faster, safer, and the most cost-effective way to land in Mexico.
By operating under a shelter, your company takes advantage of the shelter’s already existing permits, certifications, legal structure, and local administrative support staff. Not only are the liabilities and risks limited when under a shelter company, but the annual cost to operate is significantly lower than running an operation as a standalone independent corporation. The entire responsibility for your operation falls on the shelter company provider, from facility permitting, legal and fiscal obligations, to labor law and environmental law compliance. Using a shelter company provider can save newly-landed companies anywhere from 12–25 percent in annual operational costs for facilities with 50-100 employees.
“We considered a number of options, including the possibility of opening our own facility or utilizing a shelter service. We considered locations such as Mexicali, Ciudad Juarez, Rosarito, and Tijuana. We chose Tijuana for its proximity to our Irvine, California Headquarters, the quality of the workforce in Tijuana and the ability to work with a shelter company provider,” said John Hamilton, Aspen Medical Products’ vice president of strategic initiatives and special projects. The California-based Aspen Medical Products is a leader in spinal bracing products and orthotics.
Working with a shelter company provider allows the manufacturer to focus 100 percent of their efforts on the manufacturing side while the provider handles all the background details. The manufacturer is still involved in major decisions, especially in regard to site selection, build out, and recruiting qualifications. Companies that utilize a shelter company provider can stay under the shelter exactly as long as they want to. Some choose to permanently remain under the shelter and others, over time, transition to their own independent corporation while still utilizing the local administrative support offered by the provider.
The Independent Mexican Corporation
Starting off with an independent corporation is the second most popular method. Establishing your independent corporation means setting up your own Mexican corporate entity and obtaining your own permits, such as the IMMEX certification for tariff-free temporary imports. Firms that offer shelter company services will usually offer this option as well, allowing manufacturers to establish their own business entity while providing support along the way.
The ramp-up time is longer and the exposure greater, though often a choice for manufacturers with 500 employees or more and those wishing to establish a long-term presence in the region. Additionally, some OEMs just prefer to have their own Mexican corporation. Manufacturers who choose the independent corporation option will often work with local shelter company firms, taking advantage of their team’s administrative, legal, HR, and tax expertise familiar with both U.S. and Mexican laws.
The medical device manufacturing industry in Mexico is not a passing fad, but rather a robust, well-established and well-supported mega-region. With the global medical device market expected to reach $532.2 billion by 2024, will your company join the ranks of top medical device manufacturers in Mexico?
Kate Reifers is a writer and researcher for Co-Production International, specializing in manufacturing in Mexico and Baja California.




























