Tim Erwin, Technical Advisor, NPI/Medical04.03.17
The first example of silicone rubber was produced as early as 1872. The substance was very viscous oil, developed by reacting diethoxydiethysilane with water and trace amounts of acids. The first commercial grades were produced in 1943, only high-consistency rubbers (HCRs), by the Dow Chemical Company, with many other companies following shortly thereafter. It was not until the late1970s that a “pumpable” silicone rubber was developed and available to fabricators. Since then, LSRs have continued to evolve and surpassed the use of HCRs for injection molding. Liquid silicone rubber (LSR) has come a long way and today, is utilized by a variety of industries, from automotive to medical.
When someone mentions using a silicone product, one or more of several varied products could come to mind. Although all trace back to the common element silica, their forms and end uses are as varied as their processes. Thermoset elastomers can basically be broken out into two types:
HCR/HCE
LSR/LIM
Terminology & Description
Silicone elastomers (methyl-silicone, vinyl-methyl-silicone, fluoro-vinyl-methyl-silicone, and phenyl-vinyl-methyl-silicone) are formulated from the second most abundant element on earth—silica. Silica is a major component of many clays, sand, rocks, and quartz. If modified by adding oxygen, silica becomes silicon, another gray, rocklike substance. Perhaps most famous for revolutionizing the electronics industry (Silicon Valley, telecommunications, computers, electronics), silica is the base component that is processed, catalyzed, and chemically processed into the components of what will become flexible LSR. Additionally, LSRs readily accept a number of fillers to provide selected physical properties, improve strength, and increase viscosity. Some are self-extinguishing or flame resistant while others are not. Following are brief descriptions of the five families of silicone products.
Processing & Production
Process
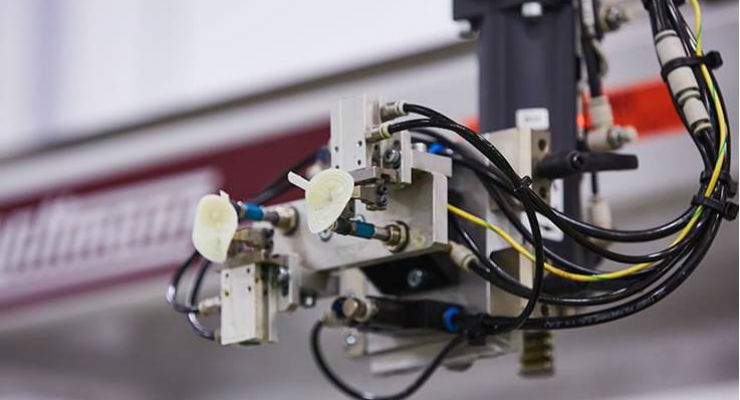
High consistency compounds are typically compression or transfer molded, thus largely limiting the design of products to shallow/simple or no details perpendicular to the parting line. High consistency rubber injection molding is possible; however, this is a limited, niche technology. Processing LSR elastomers, on the other hand, requires only three steps: meter-mixing, molding, and finishing. LSR elastomers have excellent flow qualities, and are rugged and pliable after curing in the mold, permitting extraordinary details and undercuts virtually impossible in other injection molding materials such as plastics or thermoplastic elastomers. LSRs are most commonly injection molded using injection molding machines similar to but not the same as thermoplastics. The “A” and “B” LSR components are most commonly packed and handled in 19.9 kg. (about 5 gallon) pails or 204.1 kg. (about 55 gallon) drums. The drum kits or pail kits are placed in high pressure pumps fitted with precise follower plates to push the viscous components from the drum through a precision, high pressure control pump that can be either a fixed 1:1 ratio or variable ratio type. Smaller pumps are available that can be fed by several sizes of cartridges that are popular for prototyping and product development.
The “A” and “B” components are fed through high pressure hoses from the drums through the pump(s) up to a mixing head where the two parts are brought together and then mixed in a static mixer into a homogenous mix in preparation on forming the final product. At the point in the system where the two components come into contact and blended together, a cooling medium such as chilled water ensures the mix is maintained at a controlled, cool temperature (10 to 20ºC) to inhibit the cross linking or curing reaction. The cooled LSR mix will remain stable for extended periods of up to several days.
Production
The two most common types of molding machines are reciprocation screw and several variations of screw over plunger. One uses a reciprocation screw to prepare a dose and to inject the LSR, and the other uses a feeding screw to fill an exact dosing plunger that, in turn, injects the LSR. Unlike a thermoplastic screw with several zones to compress and heat plastic by friction and to push the melt in front of the check ring, LSR screws rely on a combination of the screw flights and the LSR pump to push the mix in front of the check ring or into the plunger. Also, unlike a thermoplastic molding machine that uses heater bands to assist in plasticizing plastic pellets into a melt for injection into mold cavities, LSR machines use water jackets to keep the machine barrels and screws cool to retard the cross-linking process. If the LSR molding machine is using a shut-off nozzle to positively cut off LSR flow, it too will be cooled by a water jacket.
Silicone molds look “similar” to thermoplastic injection molds but they really are quite different. In theory (and in function) LSR molds are more closely related to compression rubber molds; rubbers and LSRs can flash (escape) through gaps as small as 0.0025 mm (0.0001 in.) and mold component fits and tolerances are an order of magnitude smaller and tighter than those normally associated with thermoplastics. Additionally, unlike the majority of plastic molds that control processing temperatures (0 to 150ºC) with water or fluids (such as glycol or thermal transfer fluid) to provide a suitable means to “cool” the plastic melt into a hardened, rigid plastic product, LSR molds are heated [usually by heating cartridges to a high temperature (150 to 200ºC)] to cure the LSR into a finished, resilient product. Thermoplastic molds commonly use ejector pins to push (eject) the finished parts out of mold cavities. The high flowing characteristic of LSRs prevents the use of the same, relatively inexpensive ejector pins. Ejector pins, if used in an LSR mold, are special designed to fit an application, usually with tight fitting angled mating faces to prevent the LSR from fouling the pin shaft. Ejection is usually using automated fixtures, sweepers, or robots to push, scrape, or pull the finished part from a core, cavity, and retainer plate or undercut mechanism.
Benefits of LSR
Applications
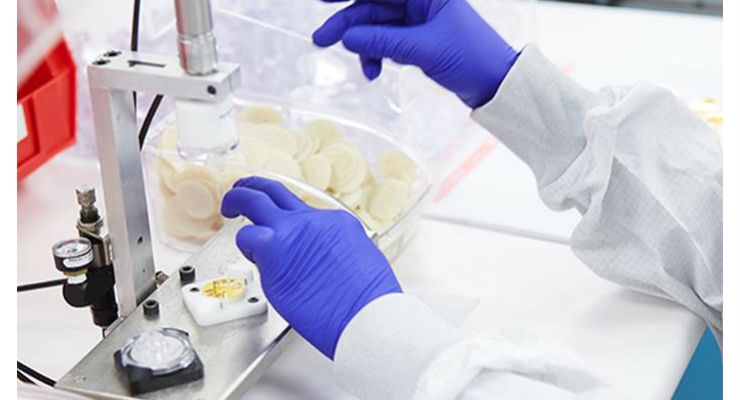
Liquid silicone rubber applications involve products that require high-precision performance, reliability, and a smooth surface. Examples include seals, sealing membranes, electric connectors, multi-pin connectors, medical applications, infant products (e.g., bottle nipples, pacifiers, and overlay on feeding spoons), and kitchen utensils (e.g., spatulas, spoons, baking pans, etc.).
Silicone rubber is often over-molded onto other parts made of different, harder, less flexible materials such as thermoplastics or metals. For example, a silicone, soft-grip handle might be over-molded onto a Nylon housing.
Since silicone rubber has many positive properties, new material advances continue to be made. Most recently, the healthcare industry is benefitting from the high optical transparency and excellent physical stability to produce products such as catheters, wound drains, endoscopic devices, hydrocephalic shunts, pacemaker lead coverings, replacement finger joints, and respiratory components.
Conclusion
The selection of the right type of silicone rubber elastomers for a specific use is largely a matter of personal preference and availability of equipment. There is little observable difference between peroxide-cured, high-consistency, silicone rubbers; addition-cured high-consistency, silicone rubbers; and liquid silicone rubbers in terms of physical property performance. The materials differ significantly, however, in terms of the processing necessary to fabricate medical devices and components. High consistency or liquid silicones are acceptable for injection molding machines. For facilities already processing high consistency elastomers, continuing with the same type of material may be the most efficient and cost-effective course of action. However, new operations entering the marketplace should give serious consideration to using liquid silicone rubber, because the capital costs and labor involved are significantly lower than those associated with the processing of a high consistency material.
Tim Erwin is a technical advisor with NPI/Medical, a company that helps medical device, life science, and healthcare OEMs accelerate their new product introductions while redefining their supply chain through a full range of prototype to production services including DFM, LSR tooling and molding, low-volume prototype tooling, bridge tooling, and production molding in an ISO 13485 environment. Headquartered in Ansonia, Conn., NPI/Medical operates out of a 66,000-square-foot manufacturing facility designed to accelerate the new product introduction (NPI) lifecycle from concept development to production launch. Find out more about the company at www.npi-med.com.
When someone mentions using a silicone product, one or more of several varied products could come to mind. Although all trace back to the common element silica, their forms and end uses are as varied as their processes. Thermoset elastomers can basically be broken out into two types:
HCR/HCE
- HCR (heat cured rubber)
- HCE (heat cured elastomers)—Very high viscosity [greater than 50,000,000 centipoises (cps)], non-flowing rubber compounds typically compression or transfer molded or extruded
LSR/LIM
- LIM (liquid injection)
- Molded or LSR (liquid silicone rubber) are lower viscosity, two-component, flowable pastes in the typical viscosities from 10,000 to 1,000,000 cps.
Terminology & Description
Silicone elastomers (methyl-silicone, vinyl-methyl-silicone, fluoro-vinyl-methyl-silicone, and phenyl-vinyl-methyl-silicone) are formulated from the second most abundant element on earth—silica. Silica is a major component of many clays, sand, rocks, and quartz. If modified by adding oxygen, silica becomes silicon, another gray, rocklike substance. Perhaps most famous for revolutionizing the electronics industry (Silicon Valley, telecommunications, computers, electronics), silica is the base component that is processed, catalyzed, and chemically processed into the components of what will become flexible LSR. Additionally, LSRs readily accept a number of fillers to provide selected physical properties, improve strength, and increase viscosity. Some are self-extinguishing or flame resistant while others are not. Following are brief descriptions of the five families of silicone products.
- Silanes and coatings are single component products that can be used to promote adhesion to substrates, crosslink plastic polymers to improve their performance properties, scavenge water to prevent premature curing of compounds and improve stability, and as a coupling agent to bind organic polymers to mineral or siliceous fillers for improved bonding and strength.
- Silicone fluids are commonly used in high performance lubricating applications involving extreme high or low temperatures. They can also be used as an additive to enhance the performance of greases, pastes, and other lubricants.
- Silicone sealants come in one- or two-component forms. The two-component form requires the end user to measure and mix the two parts together just prior to using and can be a bit tricky to use. The more common one-component form starts to cure as soon as it is exposed to air (actually the moisture in the air) and must be applied fairly quickly. These are commonly used as automotive sealants or gaskets, moisture barriers for kitchen or bathroom fixtures, and for sealing windows and doors.
- Silicone elastomers (LIM or LSR) are castable or moldable thermoset polymers with a broad range of characteristics and end uses. They are two-component resins (commonly called “A” and “B” component) that are stable for long periods of time when kept separate and sealed. They too begin to catalyze (set or harden) when mixed, but can have extended pot lives (time they can be applied, formed, or used) of up to several days at room temperatures—even longer if kept cool or cold—but set quickly at elevated temperatures (seconds at 150-180ºC). They exhibit an extended range of qualities, characteristics, and appearance, and are used for an array of products from baby bottle nipples, oven bakeware non-stick liners, healthcare or medical products, valves, and high friction grips on tools and appliances.
- R.T.V. (room-temperature vulcanizing) are two-component silicones that, when mixed together, begin to catalyze but have a reasonable pot life to enable them to be cast or poured to form a variety of end-use products. They are used in the mold-making industry to make rapid tooling for prototype parts, electronics industry for potting or encapsulating components for environmental protection of sensitive components, and movie/entertainment industries to make life-like characters or for special effects.
Processing & Production
Process
High consistency compounds are typically compression or transfer molded, thus largely limiting the design of products to shallow/simple or no details perpendicular to the parting line. High consistency rubber injection molding is possible; however, this is a limited, niche technology. Processing LSR elastomers, on the other hand, requires only three steps: meter-mixing, molding, and finishing. LSR elastomers have excellent flow qualities, and are rugged and pliable after curing in the mold, permitting extraordinary details and undercuts virtually impossible in other injection molding materials such as plastics or thermoplastic elastomers. LSRs are most commonly injection molded using injection molding machines similar to but not the same as thermoplastics. The “A” and “B” LSR components are most commonly packed and handled in 19.9 kg. (about 5 gallon) pails or 204.1 kg. (about 55 gallon) drums. The drum kits or pail kits are placed in high pressure pumps fitted with precise follower plates to push the viscous components from the drum through a precision, high pressure control pump that can be either a fixed 1:1 ratio or variable ratio type. Smaller pumps are available that can be fed by several sizes of cartridges that are popular for prototyping and product development.
The “A” and “B” components are fed through high pressure hoses from the drums through the pump(s) up to a mixing head where the two parts are brought together and then mixed in a static mixer into a homogenous mix in preparation on forming the final product. At the point in the system where the two components come into contact and blended together, a cooling medium such as chilled water ensures the mix is maintained at a controlled, cool temperature (10 to 20ºC) to inhibit the cross linking or curing reaction. The cooled LSR mix will remain stable for extended periods of up to several days.
Production
The two most common types of molding machines are reciprocation screw and several variations of screw over plunger. One uses a reciprocation screw to prepare a dose and to inject the LSR, and the other uses a feeding screw to fill an exact dosing plunger that, in turn, injects the LSR. Unlike a thermoplastic screw with several zones to compress and heat plastic by friction and to push the melt in front of the check ring, LSR screws rely on a combination of the screw flights and the LSR pump to push the mix in front of the check ring or into the plunger. Also, unlike a thermoplastic molding machine that uses heater bands to assist in plasticizing plastic pellets into a melt for injection into mold cavities, LSR machines use water jackets to keep the machine barrels and screws cool to retard the cross-linking process. If the LSR molding machine is using a shut-off nozzle to positively cut off LSR flow, it too will be cooled by a water jacket.
Silicone molds look “similar” to thermoplastic injection molds but they really are quite different. In theory (and in function) LSR molds are more closely related to compression rubber molds; rubbers and LSRs can flash (escape) through gaps as small as 0.0025 mm (0.0001 in.) and mold component fits and tolerances are an order of magnitude smaller and tighter than those normally associated with thermoplastics. Additionally, unlike the majority of plastic molds that control processing temperatures (0 to 150ºC) with water or fluids (such as glycol or thermal transfer fluid) to provide a suitable means to “cool” the plastic melt into a hardened, rigid plastic product, LSR molds are heated [usually by heating cartridges to a high temperature (150 to 200ºC)] to cure the LSR into a finished, resilient product. Thermoplastic molds commonly use ejector pins to push (eject) the finished parts out of mold cavities. The high flowing characteristic of LSRs prevents the use of the same, relatively inexpensive ejector pins. Ejector pins, if used in an LSR mold, are special designed to fit an application, usually with tight fitting angled mating faces to prevent the LSR from fouling the pin shaft. Ejection is usually using automated fixtures, sweepers, or robots to push, scrape, or pull the finished part from a core, cavity, and retainer plate or undercut mechanism.
Benefits of LSR
- Great thermal stability
- Ability to resist extreme temperatures of heat and cold
- Medical and food grade compliant
- Extremely clean and free from impurities, as well as any potential biological contaminants
- Does not discolor from UV light
- Superior color stability
- Cost effective (depending on volume)
- Virtually no scrap
- Outstanding mechanical, electrical insulating properties
- Ability to repel water and form water-tight seals
- Low chemical reactivity
- Low toxicity
- Low viscosity; suitable for molding applications requiring complex, intricate molds
- Designed to be used in highly automated, closed systems with very little labor required once the system has been put into operation
Applications
Liquid silicone rubber applications involve products that require high-precision performance, reliability, and a smooth surface. Examples include seals, sealing membranes, electric connectors, multi-pin connectors, medical applications, infant products (e.g., bottle nipples, pacifiers, and overlay on feeding spoons), and kitchen utensils (e.g., spatulas, spoons, baking pans, etc.).
Silicone rubber is often over-molded onto other parts made of different, harder, less flexible materials such as thermoplastics or metals. For example, a silicone, soft-grip handle might be over-molded onto a Nylon housing.
Since silicone rubber has many positive properties, new material advances continue to be made. Most recently, the healthcare industry is benefitting from the high optical transparency and excellent physical stability to produce products such as catheters, wound drains, endoscopic devices, hydrocephalic shunts, pacemaker lead coverings, replacement finger joints, and respiratory components.
Conclusion
The selection of the right type of silicone rubber elastomers for a specific use is largely a matter of personal preference and availability of equipment. There is little observable difference between peroxide-cured, high-consistency, silicone rubbers; addition-cured high-consistency, silicone rubbers; and liquid silicone rubbers in terms of physical property performance. The materials differ significantly, however, in terms of the processing necessary to fabricate medical devices and components. High consistency or liquid silicones are acceptable for injection molding machines. For facilities already processing high consistency elastomers, continuing with the same type of material may be the most efficient and cost-effective course of action. However, new operations entering the marketplace should give serious consideration to using liquid silicone rubber, because the capital costs and labor involved are significantly lower than those associated with the processing of a high consistency material.
Tim Erwin is a technical advisor with NPI/Medical, a company that helps medical device, life science, and healthcare OEMs accelerate their new product introductions while redefining their supply chain through a full range of prototype to production services including DFM, LSR tooling and molding, low-volume prototype tooling, bridge tooling, and production molding in an ISO 13485 environment. Headquartered in Ansonia, Conn., NPI/Medical operates out of a 66,000-square-foot manufacturing facility designed to accelerate the new product introduction (NPI) lifecycle from concept development to production launch. Find out more about the company at www.npi-med.com.