Christopher Delporte02.26.07
Case Study: Partners in the Process
An OEM Searches for a Contract Manufacturer With the Perfect Fit
Christopher Delporte
Group Editor
Perhaps one of the biggest challenges for medical device manufacturers—regardless of size—is to design, develop and deliver safe and effective devices to market in as timely a manner as possible, while at the same time keeping costs in check. The burden—financially and procedurally—can be particularly onerous for small and midsize firms, which often lack the infrastructure or manufacturing expertise to complete a project in-house from start to finish.
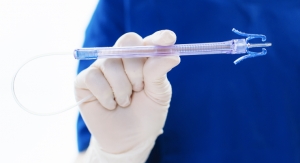
For many years, MedPro has used contract manufacturing to bring its line of needle safety devices to market. With its latest endeavor, a blood collection system, the company looked for a partner with the right combination of skills. Photo courtesy of MedPro. |
So what are the next steps? How does the device move from theory to manufactured reality and, ultimately, into the hands of healthcare professionals?
To help answer many of these questions, medical device companies often turn to contract manufacturing partners. There probably are as many outsourcing options as there are new device ideas, and today’s outsourcing companies can provide everything from individual component manufacturing to one-stop-shop service that takes a product from drawing board to packaging.
When Walter Weller, president and chief operating officer of MedPro Inc., a manufacturer of needle safety devices, was preparing to launch one of his company’s newest products—a blood collection system—he faced the same question: What’s the best way to manufacture my product?
Knowing Your Niche
No stranger to contract manufacturing, Weller said MedPro has used contract manufacturing from day one.
“We have outsourced almost exclusively all of our design, engineering and manufacturing, both onshore and offshore, for several of the products we’ve worked on,” he explained. “Our philosophy is that we are an emerging small company that has taken a rifle shot into a specific type of technology for needlestick safety, and, as a result, it would have been financially difficult to start to build internal assets for design and manufacturing.”
Lexington, KY-based MedPro started 12 years ago with a needle-destruction device, which it still produces. Following the federal Needlestick Safety and Prevention Act of 2000, the company began to realize more market opportunity as the healthcare industry responded to stricter regulations in preventing accidental needlestick injuries.
“A contaminated sharp could certainly be a lethal weapon,” Weller said.
Statistics from the Occupational Safety and Health Administration (OSHA) support bare him out. According to OSHA, healthcare workers suffer between 600,000 and one million injuries from conventional needles and sharps annually, which can lead to life-threatening infections such as hepatitis C and HIV.
“The market was moving toward passive technology very quickly,” Weller recalled. “Passive means the device requires little, if any, interaction from the needle administrator [the person giving the injection] and it can automatically deploy itself and not require an action from the user.”
MedPro also manufactures a safety dental syringe and a second-generation needleless IV system that all employ passive technology.
To get input and buy-in for the newest device, MedPro received feedback from a variety of sources, including certified medical technicians, lab directors and even distributors. Weller believes the device will be well received by end users because they were involved in the design process.
“Most of our competitors will take one system and attempt to use it across a wide base of products,” he explained. “We’ve either created or acquired systems that are designed for how they’re used in the marketplace. Does it take longer? Absolutely. Does it cost more money? Absolutely. But at the end of the day, you are building a product best suited to its market segment.”
As production plans moved forward for the blood collection system, Weller began looking for a “new approach” from a contract service provider that shared an ethic more along the lines of his own company’s philosophy. He added that the “partner” versus a “vendor” mentality was critical in the development of the product, citing speed to market, automation expertise and manufacturability as key goals.
“I feel the marketplace, traditionally, has been dominated by very large [contract manufacturing] companies; and I think smaller groups can turn much faster than larger groups can,” Weller said. “We had gone with completely integrated companies on other projects, but with this product we were looking at quicker reaction time and more direct management expertise at different stages. When we used a very large, fully integrated company, we had a program manager and everything would percolate through him as opposed to having a team approach.”
The Horizontal Solution
Weller said he found the right combination of agility, ability and experience with Integrated BioSciences, Inc. (IBS) in Lewisberry, PA. He learned about the company after doing extensive research, as well as based on a recommendation from one of IBS’s clients whom Weller knows.
The horizontal integration that IBS offered was one of the major factors in his decision “without a doubt,” Weller said. “I had very little interest in trying to re-develop production technology that already was out there. Our expectations are very high, and what [IBS has] been able to accomplish not only with us, but also with other clients, speaks very highly of what their efficiencies and strengths are. I didn’t want to show up, hand over a design, and say ‘build it for me’ and then walk away.”
Horizontal integration, in this case, involves a company that relies on a specific group of core competencies and then creates partnerships with other suppliers to fill in the gaps. The result is a start-to-finish manufacturing process that IBS controls for its clients. Rather than a vertically integrated company that offers a complete menu of services under one corporate umbrella, horizontal integration allows varied suppliers—managed by one firm—to provide their expertise based on client needs.
“We specialize in automation, assembly processes and design for manufacture,” explained Ed Paukovits, president of IBS, which started four years ago manufacturing syringes for medial device company B.Braun. “We don’t specialize in molding, for example, so, therefore, we generate relationships with a number of different molding companies—ranging from small to large—who are well recognized in their field to supplement our resources and our technology. We want people who are pushing state-of-the-art processes, and we’ve actually partnered with some automation companies because they have some capabilities we may not have. We trust our partners’ technology.”
Daniel Adlon, IBS’s vice president of strategic business development, said the strategy centers around using the best of what each supplier has to offer.
“Not every company can have a core competency that covers the gamut of product requirements; I don’t care what they say,” Adlon said. “Focus on bringing people to the table who have the other skill sets you need.”
Paukovits said this approach is the cornerstone of his company’s service to its customers, which range from medical device start-ups to multibillion-dollar corporations.
“We want to make the process transparent to the customer,” he said. “They don’t deal with 10 different companies; they deal with us. We’re the program managers. We take care of it. All the quality assurances come from IBS. It’s our responsibility. So they’re dealing with one company as if we are vertically integrated.”
According to Weller, IBS brought value from the very beginning. Though he had preliminary design engineering and prototypes for the blood collection device complete, the final design was not yet set in stone when he initially sat down with IBS.
“They brought ideas to us about how the process was structured and how things could be done a little differently for manufacturability and how it would increase quality, reduce risks and keep costs down,” he said. “IBS’s emphasis on design for manufacturability and involvement early with our design engineering helped us create a more efficient process and provided several options for how the product would be built and how its features were going to work.”
Design for manufacture is a cost driver that should not be ignored but all too often is overlooked, according to Paukovits, who added that some of the best known everyday products—such as the Ford Taurus—were designed with that philosophy in mind.
“Because 80% of the cost of manufacturing is put into the product in the design phase, we work heavily with design,” he explained. “A firm may have designed the perfect product, but they wait too long to pull in someone with design-for-manufacture experience and then we have to tell them it isn’t going to work the way it’s designed.”
From design help through production tooling, which took about a year, Weller estimates that he was able to get his new product launched in 60% of the time his company ordinarily would have taken using other methods.
“We’ve done our own external focus groups, product analysis and risk analysis on the products, but getting different sets of disciplines involved has expended our base and given us far more feedback collectively than we would have developed if these had been all internal assets,” Weller explained. “One of the great things about outsourcing with IBS is that there is no agenda but to do this as efficiently and effectively as possible, which allows us to penetrate the market and build volume. They share the same passion we have about putting these products in play.”
One of IBS’s horizontal integration partners echoed Weller’s comments.
“They don’t look at suppliers as a necessary evil. We’re someone they can work with to make a project successful,” said Mark Halstead, director of business development for Tessy Plastics, an injection molder based in Elbridge, NY. “Their definition of what they need from us is very clear. When they give you specifications, you know immediately what’s expected from you. Some people can be evasive about standards. There’s no hidden agenda. It certainly is more of a partnership mentality.”
At one point during the process, Weller explained, IBS intervened to help save MedPro time and money. “That sold me,” he said.
One of the product features that MedPro’s product design engineers had settled on would have resulted in higher production costs because additional tools would be needed to build the device. The more expensive tooling option was replaced at the insistence of IBS.
“Instead of looking for more expensive tooling for their margins, they looked at it as a partner would,” Weller said. “It wasn’t about a higher-cost or lower-cost tool; they wanted to build the perfect tool.”
Based on his recent experience with IBS, Weller said the two companies are slated to work together again in the near future and that MedPro plans to expand its product offering significantly during the next three years.
“We anticipate a very aggressive rollout schedule for 2007 and through the first quarter of ’09, and I want to make sure everything gets done appropriately,” he said. “I fully anticipate bringing [IBS] as many of our systems as they can handle.”